How does a pH indicator work? Every one of us has come across a pH based experiment. My first one was the cabbage pH colour change when I was at a nerdy friend’s birthday party in 3rd grade. Most of us, though, are directed to dip the pH indicator strip into a liquid to see what colour the paper changed in middle school. Yet, if you’re like me you never asked this question until you were either prompted by your curiosity, the homework, or a child. In my case it was a student that got me asking what is litmus paper and how do ph indicators work.
What is a pH indicator, really?
To answer this, we have to get some vocabulary out of the way.
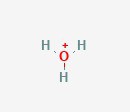 First, is hydronium (H3O+), which also goes by oxonium and a couple more synonyms. You’ll see that it is visually looks like water. Water has one oxygen and two hydrogen, whereas hydronium has three hydrogen. This also has a positive net charge. This extra hydrogen is the secret here, as that means it has an extra hydrogen atom to donate to the solution. When a solution has extra hydrogens floating around, it’s acidic.
First, is hydronium (H3O+), which also goes by oxonium and a couple more synonyms. You’ll see that it is visually looks like water. Water has one oxygen and two hydrogen, whereas hydronium has three hydrogen. This also has a positive net charge. This extra hydrogen is the secret here, as that means it has an extra hydrogen atom to donate to the solution. When a solution has extra hydrogens floating around, it’s acidic.
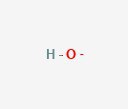 Now we look at hydroxide (OH–). This has one oxygen and one hydrogen. It’s also has a negative net charge. If a substance is basic, it is because there are many hydroxide molecules floating around in the solution.
Now we look at hydroxide (OH–). This has one oxygen and one hydrogen. It’s also has a negative net charge. If a substance is basic, it is because there are many hydroxide molecules floating around in the solution.
I keep using the word solution. The reason for this is because many hydronium and hydroxides are released in a solution, which is what gives it the ability to be detected as either acidic or basic. Solutions are when a substance is dissolved in a liquid, such as water.
All that leads to what a pH indicator is. It’s simply a way to count the number of hydronium (H3O+) or hydroxides (OH–) that a solute has to donate to the solution.
How a pH indicator works
A pH indicator is a measurement technique that uses a buffering technique to count the number of hydronium and/or hydroxides that a substance has to donate to the solution. It’s not straight counting, though. It’s logarithmic, which is counting exponentially.
As the substance or sample is dissolved, it releases either hydrogen, hydronium, or hydroxides. These are absorbed by the pH indicator, which react with the number of molecules in the solution to change colour. The more hydronium, the more acidic. The more hydroxide, the more basic.
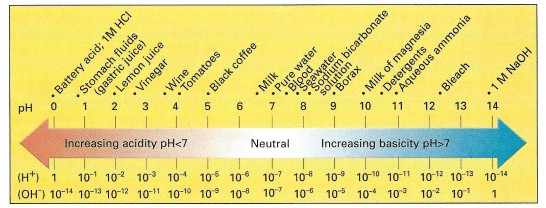
Please note that the pH scale counts hydrogen ions as well. This comes from the extra hydrogen in the hydronium.
Why pH matters
pH is a characteristic of many chemicals, much like it’s colour, smell, and reactivity. However, as you can see from the pH scale above, their are numerous substances with pH. The pH often helps us us create reactions from different chemicals, manipulating them for our uses. Changing the pH of a substance can cause the substance to change, by allowing it to react in different ways with the environment.
In my grad studies I created a basic solution to bind the beryllium-10 I was isolating to count. In the home we use bleach to kill a variety of germs that might be lurking on our counters. We use soap to bind to and loosen grease from our dishes. Vinegar has a quite useful property of removing soap scum by neutralising it.
This is one of the easiest characteristics to manipulate for a astounding array of effects.
Some experiments
Any chemistry book or boxed lab will have ideas of how you can use pH to further your child’s education and just to play with it. Below is a live workshop we did to expose secret messages with baking soda and cranberry juice.
 And get more free resources from our library while you’re at it.
And get more free resources from our library while you’re at it.
Drop your email below to get the depth of learning checklist we use AND a few suggestions on how to focus activities for levels of learning.
