Thermal expansion is the tendency of matter to change in shape, volume, and area in response to a change in temperature. It can really put a damper on how you expect things to work if you’re not expecting how the materials will react. We expect eggs to inflate and expand while being baked – that’s how delicious soufflés like dutch babies are made. We don’t necessarily expect that thermal expansion could be have disastrous failures if not accounted for – like when water freezes, expands, and bursts the soda you had cooling in the freezer.

Thermal wha….?
Thermal expansion is a physical feature of materials that is a fundamental strand in physics, the history of chemistry, and science in general. Owing to the relevance of thermodynamics in much of science and technology, it’s finely woven with the developments of classical mechanics, quantum mechanics, magnetism, and chemical kinetics. It’s applied fields such as meteorology, information theory, biology, on top of the obvious technological developments such as the steam engine, internal combustion engine, cryogenics and electricity generation.
Thermal expansion is a tiny part of the study of field of thermodynamics. Many students study this as part of Advanced Placement Physics, as well. To truly understand this topic in depth, you’d want to study thermodynamics in all it’s glory. For this article we’ll keep it simple and skim the basics, though.
The role of temperature
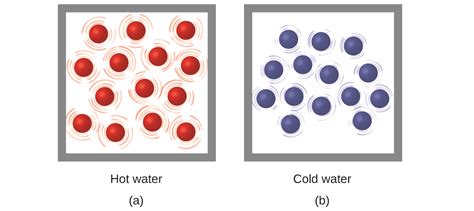
Temperature is a monotonic function of the average molecular kinetic energy of a substance.
What’s that mean? Well, a monotonic function is a function which is either entirely non-increasing or non-decreasing. A function is monotonic if its first derivative (which need not be continuous) does not change sign.
All molecules have molecular energy, or the way the atoms vibrate within them. A solid, such as the table or desk, the molecules are packed together and vibrating gently, but fast as they bounce off each other. It would be hard to see the change in molecules.
However, when you breathe, the gas leaving your lungs is also made up of molecules that float about and expand in the air. Blow up a balloon to contain them, then you can move the balloon into a cold environment where the molecules slow down causing the balloon to shrink. Move it to a warmer environment and the balloon swells again as the molecules speed up. You could, potentially, even explode the balloon if the molecules are sped up fast enough and the rubber making up the balloon is weak enough.
Dance, molecules, dance
The kinetic energy of the molecules of a substance increases when the substance is heated. The molecules start to vibrate and move more and usually maintain a greater average separation. The degree of expansion divided by the change in temperature is called the material’s coefficient of thermal expansion. It is found to generally vary with temperature.
Every material has a different coefficient, which can be looked up as you need them . However, the fact that every material has a different coefficient makes life interesting.
For a real life example, if you want to evaporate water out of milk to create a cream or condensed milk, you are using the fact that water has a very low coefficient compared to the buttermilk and cream.
On the other hand, metal alloys could have a critical failure if coefficients between the two aren’t matched well. On the other hand, understanding properties thermoset resins, polymers that are chemically set and undergo a permanent structure change with heat, can mean a long lasting product .
Not all thermal expansion is equal.
Up till now we’ve been talking about expansion. But expansion can be positive (when molecules spread apart) or negative (contraction and shrinkage). Negative thermal expansion happens to some materials when they are within a specific range of temperatures. Remember the water we mentioned earlier? As water is cooled to 4 degrees C, its coefficient of thermal expansion drops to zero, and it becomes negative when temperature is further reduced .
Beyond these basics of temperature, studies on corrosion in alloys indicates that thickness of the materials may play a role as well . This makes sense as it takes time for temperature permeate any material, so the outside may expand as it heats up sooner. This is actually the science behind bi-metal strips, such as the one inside your toaster that turns off the machine when it reaches the set temperature.
There are some things we’d never want to budge or change. Can you imagine sitting on lawn furniture that warps with your body heat? Thankfully, studies have been done on plastics, wood fibers, and composites of both so that we can have stable chairs that can withstand a wide range of temperatures without expanding and contracting with the times.
And get more free resources from our library while you’re at it.
 Drop your email below to get the hands on science lab printable you can use over and over again AND a few suggestions on how to focus activities for levels of learning. You’ll also have access to our entire library of resources you can use any time.
Drop your email below to get the hands on science lab printable you can use over and over again AND a few suggestions on how to focus activities for levels of learning. You’ll also have access to our entire library of resources you can use any time.
Levels of learning and application
All this is well and good, but how can you use this in your classroom?
Above is a lab we did that explores thermal expansion with building a thermostat (early thermometer). You can access that for free above or with our membership follow along in the replay. It’s great for littles to older students with, too.
Below are a list of labs, demos, and project based learning ideas you can tap into to take this to the next level based on where your students are.
- Blow up a balloon and move it from cold air (freezer or if winter outside) to warm air.
- Cap an empty plastic container tightly, then move it outside in winter. Move it back in side after the sides have collapsed to time how long it takes to expand out.
- Test tire inflation during the seasonal changes and keep a record log over a few weeks to see the changes with different temperatures.
- Play around with cement — the divisions in sidewalks are there to help prevent cracks with thermal expansion. What’s the greatest distance between segments to create this.
- Observe the world around you. How many instances of the segments can you find? (Sidewalks, railroad lines, etc.)
- Interview a contractor and ask about how thermal expansion impacts their work.
- Build a thermoset (directions and lab in the Resource Library).
- Play with bimetal jumping discs
- Dismantle an old toaster to find the heating element and timer (if it doesn’t work, you can actually buy and replace these parts).
- Create something with an automatic temperature shut off using the bimetals.
- Heat up a bottle to get the egg in and out trick
Which activity do you want to try first?
Let us know in the comments — or keep adding to the list!