Winter is a time for removing snow, and one fail safe go to is ice. Every winter the road crews bring out the ice trucks to help make driving conditions safer. Homeowners, as well, may salt their drives and sidewalks to prevent injury and accidents on their properties. Sure, we could dust the road and sidewalks with sand to help add grit and friction, but it doesn’t get rid of the dangerous ice so the problem isn’t really solved. So, why does salt work for this unique problem? The answer is in thermodynamics and how a solution can change the freezing temperature.
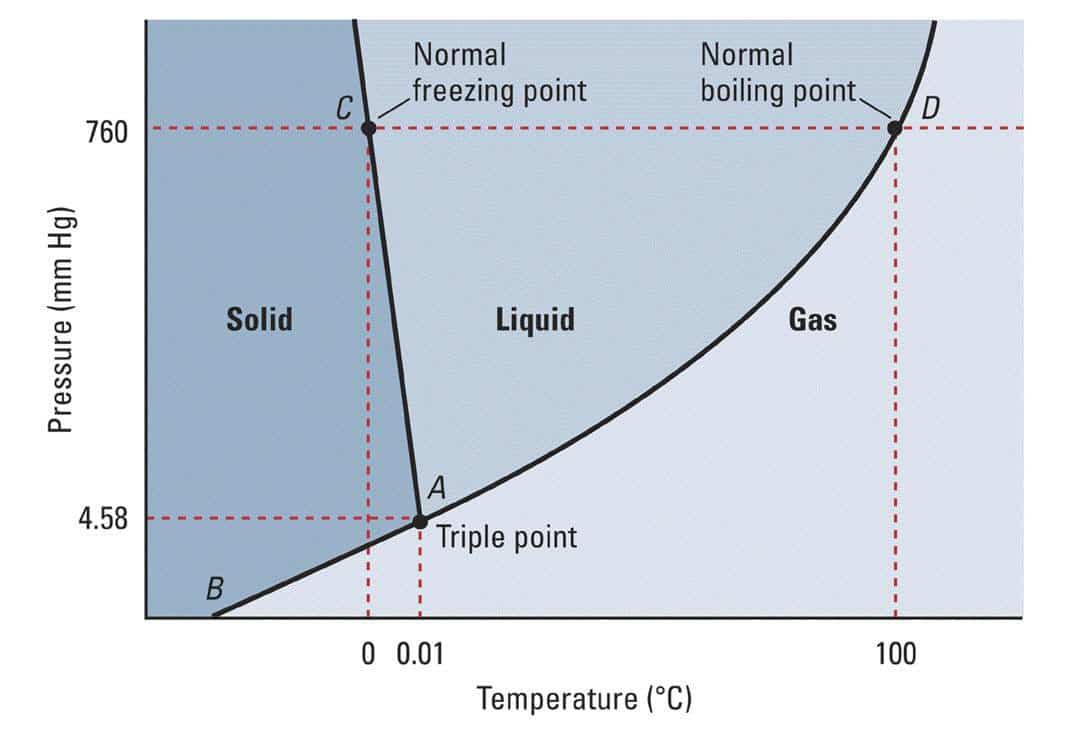
Freezing versus Melting Points Any substance has three phases: solid, liquid, and gas. These phases change with temperature and pressure, which is why water can boil under 100ºC (212ºF) at higher altitudes. The air pressure gets lower as we travel higher, and thus the boiling temperature lowers.
Simplified phase diagram of water. The lines show where the boundary between each phase ranges. At higher pressures, the phases shift to slightly different temperatures. Each substance or solution will have it’s own set of rules that make it useful for predicting and using in labs and beyond. However, when you add something to a solution, it changes to a new set of rules. This is exactly what happens when we add salt to ice: we change it’s phase diagram and set of rules it follows.
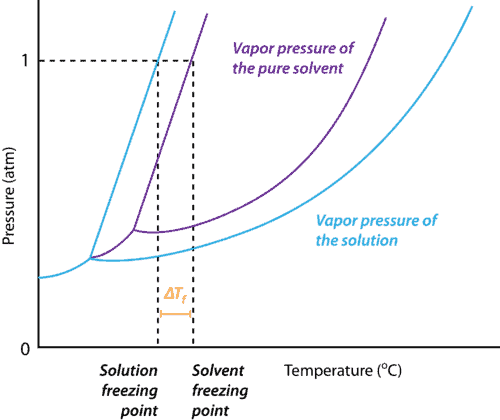
Freezing Point Depression When a solution changes, say from water to salty water, the freezing point changes until the solvent dissolves and washes away. This happens because the solvent is literally different from the original. Take a look at the graph below. The original solvent is shown in purple. It shows that at a certain temperature and pressure the solvent will be a gas, a liquid, or a solid. When any solute is added to it, the solution is fundamentally changed, so it’s graph changes. The blue line represents that change.
The vapor pressure of a solution (blue) is lower than the vapor pressure of a pure solvent (purple). As a result, the freezing point of a solvent decreases when any solute is dissolved into it. Figure from CK-12 Foundation – Christopher Auyeung. Too Cold Is Too Cold Is there such a thing as too cold for rocks? For rock salt to be halite, no, it can’t be too cold. It can, however, be too cold for the solution of water and salt to create the freezing point depression.
Moderation, As Always, Is Key Like all other things in life, you can have too much of a good thing. In this case, the salt leaches into the ground and water. The salt used on roads and sidewalks isn’t purified like the salt we use for food, so sodium, chloride, iron, and other impurities can leach into the ground and water supplies. Research done in New England in the Merrimack River watershed points to an average of 11% of the watersheds having an increased salinity and chlorine amount as a result of the salt leaching into the surrounding areas
Putting it into practice This is a fun party trick that you can use to teach kids about phase diagrams and why salt helps clear the roads of snow. Grab a piece of string, some ice, a glass of water, and some salt. Roll up your sleeves, and enjoy this simple party trick your little ones can show to everyone.
VIDEO
The FREE worksheet for this hands on activity can be found in our digital resource library. Not a member? You can sign up for free here:
Take this to the NEXT level
And get more free resources from our library while you’re at it. Drop your email below to get the depth of learning checklist we use AND a few suggestions on how to focus activities for levels of learning.
Bibliography
{21495:F3XPNEVI}
apa
default
asc
0
11469
%7B%22status%22%3A%22success%22%2C%22updateneeded%22%3Afalse%2C%22instance%22%3Afalse%2C%22meta%22%3A%7B%22request_last%22%3A500%2C%22request_next%22%3A50%2C%22used_cache%22%3Atrue%7D%2C%22data%22%3A%5B%7B%22key%22%3A%2269CNKVTI%22%2C%22library%22%3A%7B%22id%22%3A21495%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Daniel%20J.%20Peppe%20and%20Alan%20L.%20Deino%22%2C%22parsedDate%22%3A%222013%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%26lt%3Bdiv%20class%3D%26quot%3Bcsl-bib-body%26quot%3B%20style%3D%26quot%3Bline-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%26quot%3B%26gt%3B%5Cn%20%20%26lt%3Bdiv%20class%3D%26quot%3Bcsl-entry%26quot%3B%26gt%3BDaniel%20J.%20Peppe%2C%20%26amp%3B%20Alan%20L.%20Deino.%20%282013%29.%20%26lt%3Bi%26gt%3BDating%20Rocks%20and%20Fossils%20Using%20Geologic%20Methods%26lt%3B%5C%2Fi%26gt%3B.%20Nature%2C%20Scitable.%20%26lt%3Ba%20class%3D%26%23039%3Bzp-ItemURL%26%23039%3B%20href%3D%26%23039%3Bhttps%3A%5C%2F%5C%2Fwww.nature.com%5C%2Fscitable%5C%2Fknowledge%5C%2Flibrary%5C%2Fdating-rocks-and-fossils-using-geologic-methods-107924044%5C%2F%26%23039%3B%26gt%3Bhttps%3A%5C%2F%5C%2Fwww.nature.com%5C%2Fscitable%5C%2Fknowledge%5C%2Flibrary%5C%2Fdating-rocks-and-fossils-using-geologic-methods-107924044%5C%2F%26lt%3B%5C%2Fa%26gt%3B%26lt%3B%5C%2Fdiv%26gt%3B%5Cn%26lt%3B%5C%2Fdiv%26gt%3B%22%2C%22data%22%3A%7B%22itemType%22%3A%22webpage%22%2C%22title%22%3A%22Dating%20Rocks%20and%20Fossils%20Using%20Geologic%20Methods%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22name%22%3A%22Daniel%20J.%20Peppe%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22name%22%3A%22Alan%20L.%20Deino%22%7D%5D%2C%22abstractNote%22%3A%22Using%20relative%20and%20radiometric%20dating%20methods%2C%20geologists%20are%20able%20to%20answer%20the%20question%3A%20how%20old%20is%20this%20fossil%3F%22%2C%22date%22%3A%222013%22%2C%22DOI%22%3A%22%22%2C%22citationKey%22%3A%22%22%2C%22url%22%3A%22https%3A%5C%2F%5C%2Fwww.nature.com%5C%2Fscitable%5C%2Fknowledge%5C%2Flibrary%5C%2Fdating-rocks-and-fossils-using-geologic-methods-107924044%5C%2F%22%2C%22language%22%3A%22en%22%2C%22collections%22%3A%5B%5D%2C%22dateModified%22%3A%222025-12-27T21%3A27%3A53Z%22%7D%7D%2C%7B%22key%22%3A%22MWSV4GCP%22%2C%22library%22%3A%7B%22id%22%3A21495%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Steno%20and%20Winter%22%2C%22parsedDate%22%3A%221916%22%2C%22numChildren%22%3A0%7D%2C%22bib%22%3A%22%26lt%3Bdiv%20class%3D%26quot%3Bcsl-bib-body%26quot%3B%20style%3D%26quot%3Bline-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%26quot%3B%26gt%3B%5Cn%20%20%26lt%3Bdiv%20class%3D%26quot%3Bcsl-entry%26quot%3B%26gt%3BSteno%2C%20N.%2C%20%26amp%3B%20Winter%2C%20J.%20G.%20%281916%29.%20%26lt%3Bi%26gt%3BThe%20prodromus%20of%20Nicolaus%20Steno%26%23x2019%3Bs%20dissertation%20concerning%20a%20solid%20body%20enclosed%20by%20process%20of%20nature%20within%20a%20solid%3B%26lt%3B%5C%2Fi%26gt%3B%20New%20York%2C%20The%20Macmillan%20Company%3B%20London%2C%20Macmillan%20and%20Company%2C%20limited.%20%26lt%3Ba%20class%3D%26%23039%3Bzp-ItemURL%26%23039%3B%20href%3D%26%23039%3Bhttp%3A%5C%2F%5C%2Farchive.org%5C%2Fdetails%5C%2Fprodromusnicola00wintgoog%26%23039%3B%26gt%3Bhttp%3A%5C%2F%5C%2Farchive.org%5C%2Fdetails%5C%2Fprodromusnicola00wintgoog%26lt%3B%5C%2Fa%26gt%3B%26lt%3B%5C%2Fdiv%26gt%3B%5Cn%26lt%3B%5C%2Fdiv%26gt%3B%22%2C%22data%22%3A%7B%22itemType%22%3A%22book%22%2C%22title%22%3A%22The%20prodromus%20of%20Nicolaus%20Steno%27s%20dissertation%20concerning%20a%20solid%20body%20enclosed%20by%20process%20of%20nature%20within%20a%20solid%3B%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Nicolaus%22%2C%22lastName%22%3A%22Steno%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22John%20Garrett%22%2C%22lastName%22%3A%22Winter%22%7D%2C%7B%22creatorType%22%3A%22contributor%22%2C%22name%22%3A%22Harvard%20University%22%7D%5D%2C%22abstractNote%22%3A%22Book%20digitized%20by%20Google%20from%20the%20library%20of%20Harvard%20University%20and%20uploaded%20to%20the%20Internet%20Archive%20by%20user%20tpb.%3B%20With%20reproduction%20of%20the%20t.-p.%20and%20first%20page%20of%20text%20of%20original%20edition%3B%20%26quot%3BThe%20writings%20of%20Steno%26quot%3B%3A%20p.%20188-193%3B%20%26quot%3BBibliography%20of%20the%20prodromus%26quot%3B%3A%20p.%20194-203%22%2C%22date%22%3A%221916%22%2C%22originalDate%22%3A%22%22%2C%22originalPublisher%22%3A%22%22%2C%22originalPlace%22%3A%22%22%2C%22format%22%3A%22%22%2C%22ISBN%22%3A%22%22%2C%22DOI%22%3A%22%22%2C%22citationKey%22%3A%22%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Farchive.org%5C%2Fdetails%5C%2Fprodromusnicola00wintgoog%22%2C%22ISSN%22%3A%22%22%2C%22language%22%3A%22eng%22%2C%22collections%22%3A%5B%5D%2C%22dateModified%22%3A%222025-12-27T00%3A30%3A50Z%22%7D%7D%2C%7B%22key%22%3A%22RJI9BXCP%22%2C%22library%22%3A%7B%22id%22%3A21495%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Steno%20et%20al.%22%2C%22parsedDate%22%3A%221669%22%2C%22numChildren%22%3A0%7D%2C%22bib%22%3A%22%26lt%3Bdiv%20class%3D%26quot%3Bcsl-bib-body%26quot%3B%20style%3D%26quot%3Bline-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%26quot%3B%26gt%3B%5Cn%20%20%26lt%3Bdiv%20class%3D%26quot%3Bcsl-entry%26quot%3B%26gt%3BSteno%2C%20N.%2C%20Ferdinando%20II%2C%20G.-D.%20of%20T.%2C%20Accademia%20della%20Crusca%2C%20%20publisher%2C%20%26amp%3B%20Burndy%20Library%2C%20%20donor%20D.%20%281669%29.%20%26lt%3Bi%26gt%3BNicolai%20Stenonis%20De%20solido%20intra%20solidum%20naturaliter%20contento%20dissertationis%20prodromus%2C%26lt%3B%5C%2Fi%26gt%3B.%20Florentiae%26%23x202F%3B%3A%20Ex%20typographia%20sub%20signo%20Stellae.%20%26lt%3Ba%20class%3D%26%23039%3Bzp-ItemURL%26%23039%3B%20href%3D%26%23039%3Bhttp%3A%5C%2F%5C%2Farchive.org%5C%2Fdetails%5C%2Fnicolaistenonisd00sten%26%23039%3B%26gt%3Bhttp%3A%5C%2F%5C%2Farchive.org%5C%2Fdetails%5C%2Fnicolaistenonisd00sten%26lt%3B%5C%2Fa%26gt%3B%26lt%3B%5C%2Fdiv%26gt%3B%5Cn%26lt%3B%5C%2Fdiv%26gt%3B%22%2C%22data%22%3A%7B%22itemType%22%3A%22book%22%2C%22title%22%3A%22Nicolai%20Stenonis%20De%20solido%20intra%20solidum%20naturaliter%20contento%20dissertationis%20prodromus%2C%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Nicolaus%22%2C%22lastName%22%3A%22Steno%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Grand-Duke%20of%20Tuscany%22%2C%22lastName%22%3A%22Ferdinando%20II%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22publisher%22%2C%22lastName%22%3A%22Accademia%20della%20Crusca%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22donor%20DSI%22%2C%22lastName%22%3A%22Burndy%20Library%22%7D%2C%7B%22creatorType%22%3A%22contributor%22%2C%22name%22%3A%22Smithsonian%20Libraries%22%7D%5D%2C%22abstractNote%22%3A%22%26quot%3BEx%20typographia%20sub%20signo%20Stellae%26quot%3B%20is%20an%20imprint%20associated%20with%20the%20Accademia%20della%20Crusca%3B%20Title%20page%20printed%20in%20red%20and%20black%3B%20Engraved%20title%20vignette%2C%20with%20motto%20of%20Ferdinando%20II%3A%20Gratia%20obvia%20vltio%20quaesita%3B%20Head-%20and%20tail-pieces%3B%20initials%3B%20side-notes%3B%20Two%20folded%20leaves%20are%20inserted%20after%20the%20title%20leaf%2C%20containing%20descriptive%20letterpress%20%28%26quot%3Bexplicatio%20figurarum%26quot%3B%29%20on%20one%20leaf%2C%20and%20an%20engraved%20selection%20of%20diagrams%20numbered%201%20through%2025%20on%20the%20other%20%28facing%29%20leaf.%20According%20to%20Curtis%20Schuh%2C%20%26quot%3BThe%20plate%20was%20originally%20created%20as%20a%20large%20double%20plate%2C%20half%20engraved%20and%20half%20printed.%20However%2C%20due%20to%20its%20large%20size%20it%20is%20common%20to%20find%20it%20bound%20into%20the%20book%20as%20two%20plates.%26quot%3B%20In%20some%20copies%2C%20the%20folded%20leaves%20are%20bound%20in%20at%20the%20end%20of%20the%20volume%3B%20Signatures%3A%20pi1%20A-K%5Cu2074%3B%20Errata%20on%20page%20%5B1%5D%20%283rd%20group%29%3B%20The%20final%20page%20is%20blank%3B%20Dibner%20Library.%20Heralds%20of%20science%3B%20Burndy%20Library.%20Heralds%20of%20science%3B%20Schuh%2C%20C.%20Mineralogy%20%26amp%3B%20crystallography%3B%20SCDIRB%20copy%20H106481A%20is%20bound%20with%3A%20Redi%2C%20Francesco.%20Esperienze%20intorno%20alla%20generazione%20degl%26%23039%3B%20insetti.%20Firenze%20%3A%20All%26%23039%3B%20insegna%20della%20Stella%2C%20MDCLXVIII%20%5B1668%5D.%20Bound%20together%20subsequent%20to%20publication%3B%20SCDIRB%20copy%20has%20bookplate%3A%20Burndy%20Library%20...%20gift%20of%20Bern%20Dibner%3B%20SCDIRB%20copy%20has%20an%20old%20beige%20textured%20paperboard%20binding%20with%20smooth%20paper%20spine%3B%20with%20the%20title%20inked%20on%20the%20spine.%20There%20is%20an%20old%20shelfmark%20%28%26quot%3B168%26quot%3B%29%20for%20an%20unidentified%20former%20owner%26%23039%3Bs%20library%20at%20the%20foot%20of%20the%20spine%22%2C%22date%22%3A%221669%22%2C%22originalDate%22%3A%22%22%2C%22originalPublisher%22%3A%22%22%2C%22originalPlace%22%3A%22%22%2C%22format%22%3A%22%22%2C%22ISBN%22%3A%22%22%2C%22DOI%22%3A%22%22%2C%22citationKey%22%3A%22%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Farchive.org%5C%2Fdetails%5C%2Fnicolaistenonisd00sten%22%2C%22ISSN%22%3A%22%22%2C%22language%22%3A%22lat%22%2C%22collections%22%3A%5B%5D%2C%22dateModified%22%3A%222025-12-27T00%3A30%3A10Z%22%7D%7D%2C%7B%22key%22%3A%222SIDVJHF%22%2C%22library%22%3A%7B%22id%22%3A21495%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Murphy%20and%20Salvador%22%2C%22parsedDate%22%3A%221999-12%22%2C%22numChildren%22%3A0%7D%2C%22bib%22%3A%22%26lt%3Bdiv%20class%3D%26quot%3Bcsl-bib-body%26quot%3B%20style%3D%26quot%3Bline-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%26quot%3B%26gt%3B%5Cn%20%20%26lt%3Bdiv%20class%3D%26quot%3Bcsl-entry%26quot%3B%26gt%3BMurphy%2C%20M.%20A.%2C%20%26amp%3B%20Salvador%2C%20A.%20%281999%29.%20International%20Stratigraphic%20Guide%5Cn%26%23x2014%3B%20An%20abridged%20version.%20%26lt%3Bi%26gt%3BEpisodes%26lt%3B%5C%2Fi%26gt%3B%2C%20%26lt%3Bi%26gt%3B22%26lt%3B%5C%2Fi%26gt%3B%284%29%2C%20255%26%23x2013%3B271.%26lt%3B%5C%2Fdiv%26gt%3B%5Cn%26lt%3B%5C%2Fdiv%26gt%3B%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22International%20Stratigraphic%20Guide%5Cn%5Cu2014%20An%20abridged%20version%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Michael%20A.%22%2C%22lastName%22%3A%22Murphy%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Amos%22%2C%22lastName%22%3A%22Salvador%22%7D%5D%2C%22abstractNote%22%3A%22%22%2C%22date%22%3A%221999-12%22%2C%22section%22%3A%22%22%2C%22partNumber%22%3A%22%22%2C%22partTitle%22%3A%22%22%2C%22DOI%22%3A%22%22%2C%22citationKey%22%3A%22%22%2C%22url%22%3A%22%22%2C%22PMID%22%3A%22%22%2C%22PMCID%22%3A%22%22%2C%22ISSN%22%3A%22%22%2C%22language%22%3A%22%22%2C%22collections%22%3A%5B%22HGBDVFH2%22%5D%2C%22dateModified%22%3A%222025-12-27T00%3A23%3A52Z%22%7D%7D%2C%7B%22key%22%3A%22MTIS547J%22%2C%22library%22%3A%7B%22id%22%3A21495%7D%2C%22meta%22%3A%7B%22parsedDate%22%3A%222021%22%2C%22numChildren%22%3A0%7D%2C%22bib%22%3A%22%26lt%3Bdiv%20class%3D%26quot%3Bcsl-bib-body%26quot%3B%20style%3D%26quot%3Bline-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%26quot%3B%26gt%3B%5Cn%20%20%26lt%3Bdiv%20class%3D%26quot%3Bcsl-entry%26quot%3B%26gt%3BNORTH%20AMERICAN%20STRATIGRAPHIC%20CODE.%20%282021%29.%20%26lt%3Bi%26gt%3BStratigraphy%26lt%3B%5C%2Fi%26gt%3B%2C%20%26lt%3Bi%26gt%3B18%26lt%3B%5C%2Fi%26gt%3B%283%29%2C%20153%26%23x2013%3B204.%20%26lt%3Ba%20class%3D%26%23039%3Bzp-DOIURL%26%23039%3B%20href%3D%26%23039%3Bhttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.29041%5C%2Fstrat.18.3.01%26%23039%3B%26gt%3Bhttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.29041%5C%2Fstrat.18.3.01%26lt%3B%5C%2Fa%26gt%3B%26lt%3B%5C%2Fdiv%26gt%3B%5Cn%26lt%3B%5C%2Fdiv%26gt%3B%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22NORTH%20AMERICAN%20STRATIGRAPHIC%20CODE%22%2C%22creators%22%3A%5B%5D%2C%22abstractNote%22%3A%22The%202021%20version%20of%20the%20North%20American%20Stratigraphic%20Code%20is%20not%20a%20major%20revision.%20It%20simply%20states%20the%20changes%20mandated%20by%20the%20three%20approved%20amendments%20published%20in%20Easton%20et%20al.%20%282017%29%2C%20Brett%20et%20al.%20%282019%29%2C%20and%20Aubry%20et%20al.%20%282020%29%2C%20which%20contain%20modifications%20to%20Articles%2013%2C%2025%2C%2026%2C%2027%2C%2037%2C%2073%2C%2081%2C%2082%2C%20and%20Table%202.%20For%20completeness%2C%20the%20composition%20of%20the%20North%20American%20Commission%20on%20Stratigraphic%20Nomenclature%20has%20been%20updated%20in%20Appendix%20II%2C%20and%20citations%20to%20Notes%20and%20Reports%20of%20the%20Commission%20since%202005%20have%20been%20added%20to%20Appendix%20III.%20These%20changes%20follow%20Code%20amendment%20procedures%20as%20outlined%20in%20Article%2021.%22%2C%22date%22%3A%222021%22%2C%22section%22%3A%22%22%2C%22partNumber%22%3A%22%22%2C%22partTitle%22%3A%22%22%2C%22DOI%22%3A%2210.29041%5C%2Fstrat.18.3.01%22%2C%22citationKey%22%3A%22%22%2C%22url%22%3A%22https%3A%5C%2F%5C%2Fwww.micropress.org%5C%2Fmicroaccess%5C%2Fstratigraphy%5C%2Fissue-372%5C%2Farticle-2251%22%2C%22PMID%22%3A%22%22%2C%22PMCID%22%3A%22%22%2C%22ISSN%22%3A%221547139X%2C%202331656X%22%2C%22language%22%3A%22%22%2C%22collections%22%3A%5B%22HGBDVFH2%22%5D%2C%22dateModified%22%3A%222025-12-27T00%3A14%3A32Z%22%7D%7D%2C%7B%22key%22%3A%22YV9LWY3C%22%2C%22library%22%3A%7B%22id%22%3A21495%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Striegel%22%2C%22parsedDate%22%3A%222022%22%2C%22numChildren%22%3A0%7D%2C%22bib%22%3A%22%26lt%3Bdiv%20class%3D%26quot%3Bcsl-bib-body%26quot%3B%20style%3D%26quot%3Bline-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%26quot%3B%26gt%3B%5Cn%20%20%26lt%3Bdiv%20class%3D%26quot%3Bcsl-entry%26quot%3B%26gt%3BStriegel%2C%20A.%20M.%20%282022%29.%20Size-Exclusion%20Chromatography%3A%20A%20Twenty-First%20Century%20Perspective.%20%26lt%3Bi%26gt%3BChromatographia%26lt%3B%5C%2Fi%26gt%3B%2C%20%26lt%3Bi%26gt%3B85%26lt%3B%5C%2Fi%26gt%3B%284%29%2C%20307%26%23x2013%3B313.%20%26lt%3Ba%20class%3D%26%23039%3Bzp-DOIURL%26%23039%3B%20href%3D%26%23039%3Bhttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1007%5C%2Fs10337-022-04143-1%26%23039%3B%26gt%3Bhttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1007%5C%2Fs10337-022-04143-1%26lt%3B%5C%2Fa%26gt%3B%26lt%3B%5C%2Fdiv%26gt%3B%5Cn%26lt%3B%5C%2Fdiv%26gt%3B%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Size-Exclusion%20Chromatography%3A%20A%20Twenty-First%20Century%20Perspective%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Andr%5Cu00e9%20M.%22%2C%22lastName%22%3A%22Striegel%22%7D%5D%2C%22abstractNote%22%3A%22%22%2C%22date%22%3A%2204%5C%2F2022%22%2C%22section%22%3A%22%22%2C%22partNumber%22%3A%22%22%2C%22partTitle%22%3A%22%22%2C%22DOI%22%3A%2210.1007%5C%2Fs10337-022-04143-1%22%2C%22citationKey%22%3A%22%22%2C%22url%22%3A%22https%3A%5C%2F%5C%2Flink.springer.com%5C%2F10.1007%5C%2Fs10337-022-04143-1%22%2C%22PMID%22%3A%22%22%2C%22PMCID%22%3A%22%22%2C%22ISSN%22%3A%220009-5893%2C%201612-1112%22%2C%22language%22%3A%22en%22%2C%22collections%22%3A%5B%22XAWSHPGP%22%5D%2C%22dateModified%22%3A%222025-12-26T22%3A02%3A14Z%22%7D%7D%2C%7B%22key%22%3A%22JMQEM9DN%22%2C%22library%22%3A%7B%22id%22%3A21495%7D%2C%22meta%22%3A%7B%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%26lt%3Bdiv%20class%3D%26quot%3Bcsl-bib-body%26quot%3B%20style%3D%26quot%3Bline-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%26quot%3B%26gt%3B%5Cn%20%20%26lt%3Bdiv%20class%3D%26quot%3Bcsl-entry%26quot%3B%26gt%3B%26lt%3Bi%26gt%3BAffinity%20Chromatography%20%7C%20Principles%26lt%3B%5C%2Fi%26gt%3B.%20%28n.d.%29.%20Cube%20Biotech.%20Retrieved%20December%2026%2C%202025%2C%20from%20%26lt%3Ba%20class%3D%26%23039%3Bzp-ItemURL%26%23039%3B%20href%3D%26%23039%3Bhttps%3A%5C%2F%5C%2Fcube-biotech.com%5C%2Four-science%5C%2Fprotein-purification%5C%2Faffinity-chromatography%5C%2F%26%23039%3B%26gt%3Bhttps%3A%5C%2F%5C%2Fcube-biotech.com%5C%2Four-science%5C%2Fprotein-purification%5C%2Faffinity-chromatography%5C%2F%26lt%3B%5C%2Fa%26gt%3B%26lt%3B%5C%2Fdiv%26gt%3B%5Cn%26lt%3B%5C%2Fdiv%26gt%3B%22%2C%22data%22%3A%7B%22itemType%22%3A%22webpage%22%2C%22title%22%3A%22Affinity%20Chromatography%20%7C%20Principles%22%2C%22creators%22%3A%5B%5D%2C%22abstractNote%22%3A%22Protein%20purification%20via%20affinity%20chromatography%20is%20a%20powerful%20method.%20Find%20out%20how%20to%20choose%20the%20correct%20tag%20for%20your%20protein.%22%2C%22date%22%3A%22%22%2C%22DOI%22%3A%22%22%2C%22citationKey%22%3A%22%22%2C%22url%22%3A%22https%3A%5C%2F%5C%2Fcube-biotech.com%5C%2Four-science%5C%2Fprotein-purification%5C%2Faffinity-chromatography%5C%2F%22%2C%22language%22%3A%22en-GB%22%2C%22collections%22%3A%5B%5D%2C%22dateModified%22%3A%222025-12-26T21%3A59%3A21Z%22%7D%7D%2C%7B%22key%22%3A%22T6SFI8QB%22%2C%22library%22%3A%7B%22id%22%3A21495%7D%2C%22meta%22%3A%7B%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%26lt%3Bdiv%20class%3D%26quot%3Bcsl-bib-body%26quot%3B%20style%3D%26quot%3Bline-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%26quot%3B%26gt%3B%5Cn%20%20%26lt%3Bdiv%20class%3D%26quot%3Bcsl-entry%26quot%3B%26gt%3B%26lt%3Bi%26gt%3BIntroduction%20to%20Affinity%20Chromatography%20%7C%20Bio-Rad%26lt%3B%5C%2Fi%26gt%3B.%20%28n.d.%29.%20Retrieved%20December%2026%2C%202025%2C%20from%20%26lt%3Ba%20class%3D%26%23039%3Bzp-ItemURL%26%23039%3B%20href%3D%26%23039%3Bhttps%3A%5C%2F%5C%2Fwww.bio-rad.com%5C%2Fen-us%5C%2Fapplications-technologies%5C%2Fintroduction-affinity-chromatography%3FID%3DLUSMJIDN%26%23039%3B%26gt%3Bhttps%3A%5C%2F%5C%2Fwww.bio-rad.com%5C%2Fen-us%5C%2Fapplications-technologies%5C%2Fintroduction-affinity-chromatography%3FID%3DLUSMJIDN%26lt%3B%5C%2Fa%26gt%3B%26lt%3B%5C%2Fdiv%26gt%3B%5Cn%26lt%3B%5C%2Fdiv%26gt%3B%22%2C%22data%22%3A%7B%22itemType%22%3A%22webpage%22%2C%22title%22%3A%22Introduction%20to%20Affinity%20Chromatography%20%7C%20Bio-Rad%22%2C%22creators%22%3A%5B%5D%2C%22abstractNote%22%3A%22Affinity%20chromatography%20is%20a%20protein%20separation%20method%20based%20on%20a%20specific%20binding%20interaction%20between%20an%20immobilized%20ligand%20and%20its%20binding%20partner.%22%2C%22date%22%3A%22%22%2C%22DOI%22%3A%22%22%2C%22citationKey%22%3A%22%22%2C%22url%22%3A%22https%3A%5C%2F%5C%2Fwww.bio-rad.com%5C%2Fen-us%5C%2Fapplications-technologies%5C%2Fintroduction-affinity-chromatography%3FID%3DLUSMJIDN%22%2C%22language%22%3A%22en-us%22%2C%22collections%22%3A%5B%5D%2C%22dateModified%22%3A%222025-12-26T21%3A57%3A58Z%22%7D%7D%2C%7B%22key%22%3A%22XG7ZVESZ%22%2C%22library%22%3A%7B%22id%22%3A21495%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Yan%20et%20al.%22%2C%22parsedDate%22%3A%222024-07-23%22%2C%22numChildren%22%3A0%7D%2C%22bib%22%3A%22%26lt%3Bdiv%20class%3D%26quot%3Bcsl-bib-body%26quot%3B%20style%3D%26quot%3Bline-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%26quot%3B%26gt%3B%5Cn%20%20%26lt%3Bdiv%20class%3D%26quot%3Bcsl-entry%26quot%3B%26gt%3BYan%2C%20Y.%2C%20Xing%2C%20T.%2C%20Huang%2C%20X.%2C%20Peng%2C%20W.%2C%20Wang%2C%20S.%2C%20%26amp%3B%20Li%2C%20N.%20%282024%29.%20Affinity-Resolved%20Size%20Exclusion%20Chromatography%20Coupled%20to%20Mass%20Spectrometry%3A%20A%20Novel%20Tool%20to%20Study%20the%20Attribute-and-Function%20Relationship%20in%20Therapeutic%20Monoclonal%20Antibodies.%20%26lt%3Bi%26gt%3BAnalytical%20Chemistry%26lt%3B%5C%2Fi%26gt%3B%2C%20%26lt%3Bi%26gt%3B96%26lt%3B%5C%2Fi%26gt%3B%2829%29%2C%2011716%26%23x2013%3B11724.%20%26lt%3Ba%20class%3D%26%23039%3Bzp-DOIURL%26%23039%3B%20href%3D%26%23039%3Bhttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1021%5C%2Facs.analchem.4c00660%26%23039%3B%26gt%3Bhttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1021%5C%2Facs.analchem.4c00660%26lt%3B%5C%2Fa%26gt%3B%26lt%3B%5C%2Fdiv%26gt%3B%5Cn%26lt%3B%5C%2Fdiv%26gt%3B%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Affinity-Resolved%20Size%20Exclusion%20Chromatography%20Coupled%20to%20Mass%20Spectrometry%3A%20A%20Novel%20Tool%20to%20Study%20the%20Attribute-and-Function%20Relationship%20in%20Therapeutic%20Monoclonal%20Antibodies%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Yuetian%22%2C%22lastName%22%3A%22Yan%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Tao%22%2C%22lastName%22%3A%22Xing%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Xiaoxiao%22%2C%22lastName%22%3A%22Huang%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Wenjing%22%2C%22lastName%22%3A%22Peng%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Shunhai%22%2C%22lastName%22%3A%22Wang%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Ning%22%2C%22lastName%22%3A%22Li%22%7D%5D%2C%22abstractNote%22%3A%22Assessment%20of%20critical%20quality%20attributes%20%28CQAs%29%20is%20an%20important%20aspect%20during%20the%20development%20of%20therapeutic%20monoclonal%20antibodies%20%28mAbs%29.%20Attributes%20that%20affect%20either%20the%20target%20binding%20or%20Fc%20receptor%20engagement%20may%20have%20direct%20impacts%20on%20the%20drug%20safety%20and%20efficacy%20and%20thus%20are%20considered%20as%20CQAs.%20Native%20size%20exclusion%20chromatography%20%28SEC%29-based%20competitive%20binding%20assay%20has%20recently%20been%20reported%20and%20demonstrated%20significant%20benefits%20compared%20to%20conventional%20approaches%20for%20CQA%20identification%2C%20owing%20to%20its%20faster%20turn-around%20and%20higher%20multiplexity.%20Expanding%20on%20the%20similar%20concept%2C%20we%20report%20the%20development%20of%20a%20novel%20affinity-resolved%20size%20exclusion%20chromatography-mass%20spectrometry%20%28AR-SEC-MS%29%20method%20for%20rapid%20CQA%20evaluation%20in%20therapeutic%20mAbs.%20This%20method%20features%20wide%20applicability%2C%20fast%20turn-around%2C%20high%20multiplexity%2C%20and%20easy%20implementation.%20Using%20the%20well-studied%20Fc%20gamma%20receptor%20III-A%20%28Fc%5Cu03b3RIIIa%29%20and%20Fc%20interaction%20as%20a%20model%20system%2C%20the%20effectiveness%20of%20this%20method%20in%20studying%20the%20attribute-and-function%20relationship%20was%20demonstrated.%20Further%2C%20two%20case%20studies%20were%20detailed%20to%20showcase%20the%20application%20of%20this%20method%20in%20assessing%20CQAs%20related%20to%20antibody%20target%20binding%2C%20which%20included%20unusual%20N-linked%20glycosylation%20in%20a%20bispecific%20antibody%20and%20Met%20oxidation%20in%20a%20monospecific%20antibody%2C%20both%20occurring%20within%20the%20complementarity-determining%20regions%20%28CDRs%29.%22%2C%22date%22%3A%222024-07-23%22%2C%22section%22%3A%22%22%2C%22partNumber%22%3A%22%22%2C%22partTitle%22%3A%22%22%2C%22DOI%22%3A%2210.1021%5C%2Facs.analchem.4c00660%22%2C%22citationKey%22%3A%22%22%2C%22url%22%3A%22%22%2C%22PMID%22%3A%22%22%2C%22PMCID%22%3A%22%22%2C%22ISSN%22%3A%221520-6882%22%2C%22language%22%3A%22eng%22%2C%22collections%22%3A%5B%22XAWSHPGP%22%5D%2C%22dateModified%22%3A%222025-12-26T21%3A49%3A39Z%22%7D%7D%2C%7B%22key%22%3A%22U6XAGSRZ%22%2C%22library%22%3A%7B%22id%22%3A21495%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Gjoka%20et%20al.%22%2C%22parsedDate%22%3A%222014-12-01%22%2C%22numChildren%22%3A0%7D%2C%22bib%22%3A%22%26lt%3Bdiv%20class%3D%26quot%3Bcsl-bib-body%26quot%3B%20style%3D%26quot%3Bline-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%26quot%3B%26gt%3B%5Cn%20%20%26lt%3Bdiv%20class%3D%26quot%3Bcsl-entry%26quot%3B%26gt%3BGjoka%2C%20X.%2C%20Schofield%2C%20M.%2C%20Cvetkovic%2C%20A.%2C%20%26amp%3B%20Gantier%2C%20R.%20%282014%29.%20Combined%20Protein%20A%20and%20size%20exclusion%20high%20performance%20liquid%20chromatography%20for%20the%20single-step%20measurement%20of%20mAb%2C%20aggregates%20and%20host%20cell%20proteins.%20%26lt%3Bi%26gt%3BJournal%20of%20Chromatography.%20B%2C%20Analytical%20Technologies%20in%20the%20Biomedical%20and%20Life%20Sciences%26lt%3B%5C%2Fi%26gt%3B%2C%20%26lt%3Bi%26gt%3B972%26lt%3B%5C%2Fi%26gt%3B%2C%2048%26%23x2013%3B52.%20%26lt%3Ba%20class%3D%26%23039%3Bzp-DOIURL%26%23039%3B%20href%3D%26%23039%3Bhttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1016%5C%2Fj.jchromb.2014.09.017%26%23039%3B%26gt%3Bhttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1016%5C%2Fj.jchromb.2014.09.017%26lt%3B%5C%2Fa%26gt%3B%26lt%3B%5C%2Fdiv%26gt%3B%5Cn%26lt%3B%5C%2Fdiv%26gt%3B%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Combined%20Protein%20A%20and%20size%20exclusion%20high%20performance%20liquid%20chromatography%20for%20the%20single-step%20measurement%20of%20mAb%2C%20aggregates%20and%20host%20cell%20proteins%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Xhorxhi%22%2C%22lastName%22%3A%22Gjoka%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Mark%22%2C%22lastName%22%3A%22Schofield%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Aleksandar%22%2C%22lastName%22%3A%22Cvetkovic%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Rene%22%2C%22lastName%22%3A%22Gantier%22%7D%5D%2C%22abstractNote%22%3A%22Quantification%20of%20monoclonal%20antibody%20%28mAb%29%20monomer%2C%20mAb%20aggregates%2C%20and%20host%20cell%20proteins%20%28HCPs%29%20is%20critical%20for%20the%20optimization%20of%20the%20mAb%20production%20process.%20The%20present%20work%20describes%20a%20single%20high%20throughput%20analytical%20tool%20capable%20of%20tracking%20the%20concentration%20of%20mAb%2C%20mAb%20aggregate%20and%20HCPs%20in%20a%20growing%20cell%20culture%20batch.%20By%20combining%20two%20analytical%20HPLC%20methods%2C%20Protein%20A%20affinity%20and%20size-exclusion%20chromatography%20%28SEC%29%2C%20it%20is%20possible%20to%20detect%20a%20relative%20increase%20or%20decrease%20in%20the%20concentration%20of%20all%20three%20entities%20simultaneously.%20A%20comparison%20of%20the%20combined%20Protein%20A-SEC%20assay%20to%20SEC%20alone%20was%20performed%2C%20demonstrating%20that%20it%20can%20be%20useful%20tool%20for%20the%20quantification%20of%20mAb%20monomer%20along%20with%20trending%20data%20for%20mAb%20aggregate%20and%20HCP.%20Furthermore%2C%20the%20study%20shows%20that%20the%20Protein%20A-SEC%20method%20is%20at%20least%20as%20accurate%20as%20other%20commonly%20used%20analytical%20methods%20such%20as%20ELISA%20and%20Bradford.%22%2C%22date%22%3A%222014-12-01%22%2C%22section%22%3A%22%22%2C%22partNumber%22%3A%22%22%2C%22partTitle%22%3A%22%22%2C%22DOI%22%3A%2210.1016%5C%2Fj.jchromb.2014.09.017%22%2C%22citationKey%22%3A%22%22%2C%22url%22%3A%22%22%2C%22PMID%22%3A%22%22%2C%22PMCID%22%3A%22%22%2C%22ISSN%22%3A%221873-376X%22%2C%22language%22%3A%22eng%22%2C%22collections%22%3A%5B%22XAWSHPGP%22%5D%2C%22dateModified%22%3A%222025-12-26T21%3A39%3A13Z%22%7D%7D%2C%7B%22key%22%3A%22UE2BXCY9%22%2C%22library%22%3A%7B%22id%22%3A21495%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22London%20et%20al.%22%2C%22parsedDate%22%3A%222015%22%2C%22numChildren%22%3A0%7D%2C%22bib%22%3A%22%26lt%3Bdiv%20class%3D%26quot%3Bcsl-bib-body%26quot%3B%20style%3D%26quot%3Bline-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%26quot%3B%26gt%3B%5Cn%20%20%26lt%3Bdiv%20class%3D%26quot%3Bcsl-entry%26quot%3B%26gt%3BLondon%2C%20A.%20S.%2C%20Patel%2C%20K.%2C%20Quinn%2C%20L.%2C%20%26amp%3B%20Lemmerer%2C%20M.%20%282015%29.%20Application%20of%20coupled%20affinity-sizing%20chromatography%20for%20the%20detection%20of%20proteolyzed%20HSA-tagged%20proteins.%20%26lt%3Bi%26gt%3BProtein%20Expression%20and%20Purification%26lt%3B%5C%2Fi%26gt%3B%2C%20%26lt%3Bi%26gt%3B108%26lt%3B%5C%2Fi%26gt%3B%2C%2080%26%23x2013%3B84.%20%26lt%3Ba%20class%3D%26%23039%3Bzp-DOIURL%26%23039%3B%20href%3D%26%23039%3Bhttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1016%5C%2Fj.pep.2014.12.005%26%23039%3B%26gt%3Bhttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1016%5C%2Fj.pep.2014.12.005%26lt%3B%5C%2Fa%26gt%3B%26lt%3B%5C%2Fdiv%26gt%3B%5Cn%26lt%3B%5C%2Fdiv%26gt%3B%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Application%20of%20coupled%20affinity-sizing%20chromatography%20for%20the%20detection%20of%20proteolyzed%20HSA-tagged%20proteins%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Anne%20Serdakowski%22%2C%22lastName%22%3A%22London%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Kunal%22%2C%22lastName%22%3A%22Patel%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Lisa%22%2C%22lastName%22%3A%22Quinn%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Martin%22%2C%22lastName%22%3A%22Lemmerer%22%7D%5D%2C%22abstractNote%22%3A%22%22%2C%22date%22%3A%2204%5C%2F2015%22%2C%22section%22%3A%22%22%2C%22partNumber%22%3A%22%22%2C%22partTitle%22%3A%22%22%2C%22DOI%22%3A%2210.1016%5C%2Fj.pep.2014.12.005%22%2C%22citationKey%22%3A%22%22%2C%22url%22%3A%22https%3A%5C%2F%5C%2Flinkinghub.elsevier.com%5C%2Fretrieve%5C%2Fpii%5C%2FS1046592814002903%22%2C%22PMID%22%3A%22%22%2C%22PMCID%22%3A%22%22%2C%22ISSN%22%3A%2210465928%22%2C%22language%22%3A%22en%22%2C%22collections%22%3A%5B%22XAWSHPGP%22%5D%2C%22dateModified%22%3A%222025-12-26T21%3A37%3A25Z%22%7D%7D%2C%7B%22key%22%3A%229J7HLWKI%22%2C%22library%22%3A%7B%22id%22%3A21495%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Corso%20et%20al.%22%2C%22parsedDate%22%3A%222017-09-14%22%2C%22numChildren%22%3A0%7D%2C%22bib%22%3A%22%26lt%3Bdiv%20class%3D%26quot%3Bcsl-bib-body%26quot%3B%20style%3D%26quot%3Bline-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%26quot%3B%26gt%3B%5Cn%20%20%26lt%3Bdiv%20class%3D%26quot%3Bcsl-entry%26quot%3B%26gt%3BCorso%2C%20G.%2C%20M%26%23xE4%3Bger%2C%20I.%2C%20Lee%2C%20Y.%2C%20G%26%23xF6%3Brgens%2C%20A.%2C%20Bultema%2C%20J.%2C%20Giebel%2C%20B.%2C%20Wood%2C%20M.%20J.%20A.%2C%20Nordin%2C%20J.%20Z.%2C%20%26amp%3B%20Andaloussi%2C%20S.%20E.%20%282017%29.%20Reproducible%20and%20scalable%20purification%20of%20extracellular%20vesicles%20using%20combined%20bind-elute%20and%20size%20exclusion%20chromatography.%20%26lt%3Bi%26gt%3BScientific%20Reports%26lt%3B%5C%2Fi%26gt%3B%2C%20%26lt%3Bi%26gt%3B7%26lt%3B%5C%2Fi%26gt%3B%281%29%2C%2011561.%20%26lt%3Ba%20class%3D%26%23039%3Bzp-DOIURL%26%23039%3B%20href%3D%26%23039%3Bhttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1038%5C%2Fs41598-017-10646-x%26%23039%3B%26gt%3Bhttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1038%5C%2Fs41598-017-10646-x%26lt%3B%5C%2Fa%26gt%3B%26lt%3B%5C%2Fdiv%26gt%3B%5Cn%26lt%3B%5C%2Fdiv%26gt%3B%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Reproducible%20and%20scalable%20purification%20of%20extracellular%20vesicles%20using%20combined%20bind-elute%20and%20size%20exclusion%20chromatography%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Giulia%22%2C%22lastName%22%3A%22Corso%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Imre%22%2C%22lastName%22%3A%22M%5Cu00e4ger%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Yi%22%2C%22lastName%22%3A%22Lee%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Andr%5Cu00e9%22%2C%22lastName%22%3A%22G%5Cu00f6rgens%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Jarred%22%2C%22lastName%22%3A%22Bultema%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Bernd%22%2C%22lastName%22%3A%22Giebel%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Matthew%20J.%20A.%22%2C%22lastName%22%3A%22Wood%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Joel%20Z.%22%2C%22lastName%22%3A%22Nordin%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Samir%20El%22%2C%22lastName%22%3A%22Andaloussi%22%7D%5D%2C%22abstractNote%22%3A%22Abstract%20%5Cn%20%20%20%20%20%20%20%20%20%20%20%20Extracellular%20vesicles%20%28EVs%29%20play%20a%20pivotal%20role%20in%20cell-to-cell%20communication%20and%20have%20been%20shown%20to%20take%20part%20in%20several%20physiological%20and%20pathological%20processes.%20EVs%20have%20traditionally%20been%20purified%20by%20ultracentrifugation%20%28UC%29%2C%20however%20UC%20has%20limitations%2C%20including%20resulting%20in%2C%20operator-dependant%20yields%2C%20EV%20aggregation%20and%20altered%20EV%20morphology%2C%20and%20moreover%20is%20time%20consuming.%20Here%20we%20show%20that%20commercially%20available%20bind-elute%20size%20exclusion%20chromatography%20%28BE-SEC%29%20columns%20purify%20EVs%20with%20high%20yield%20%28recovery%20~%2080%25%29%20in%20a%20time-efficient%20manner%20compared%20to%20current%20methodologies.%20This%20technique%20is%20reproducible%20and%20scalable%2C%20and%20surface%20marker%20analysis%20by%20bead-based%20flow%20cytometry%20revealed%20highly%20similar%20expression%20signatures%20compared%20with%20UC-purified%20samples.%20Furthermore%2C%20uptake%20of%20eGFP%20labelled%20EVs%20in%20recipient%20cells%20was%20comparable%20between%20BE-SEC%20and%20UC%20samples.%20Hence%2C%20the%20BE-SEC%20based%20EV%20purification%20method%20represents%20an%20important%20methodological%20advance%20likely%20to%20facilitate%20robust%20and%20reproducible%20studies%20of%20EV%20biology%20and%20therapeutic%20application.%22%2C%22date%22%3A%222017-09-14%22%2C%22section%22%3A%22%22%2C%22partNumber%22%3A%22%22%2C%22partTitle%22%3A%22%22%2C%22DOI%22%3A%2210.1038%5C%2Fs41598-017-10646-x%22%2C%22citationKey%22%3A%22%22%2C%22url%22%3A%22https%3A%5C%2F%5C%2Fwww.nature.com%5C%2Farticles%5C%2Fs41598-017-10646-x%22%2C%22PMID%22%3A%22%22%2C%22PMCID%22%3A%22%22%2C%22ISSN%22%3A%222045-2322%22%2C%22language%22%3A%22en%22%2C%22collections%22%3A%5B%22XAWSHPGP%22%5D%2C%22dateModified%22%3A%222025-12-24T20%3A31%3A20Z%22%7D%7D%2C%7B%22key%22%3A%22IKXTUCDH%22%2C%22library%22%3A%7B%22id%22%3A21495%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Lowe%20and%20Lowe%22%2C%22parsedDate%22%3A%221985%22%2C%22numChildren%22%3A0%7D%2C%22bib%22%3A%22%26lt%3Bdiv%20class%3D%26quot%3Bcsl-bib-body%26quot%3B%20style%3D%26quot%3Bline-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%26quot%3B%26gt%3B%5Cn%20%20%26lt%3Bdiv%20class%3D%26quot%3Bcsl-entry%26quot%3B%26gt%3BLowe%2C%20C.%20R.%2C%20%26amp%3B%20Lowe%2C%20C.%20R.%20%281985%29.%20%26lt%3Bi%26gt%3BAn%20introduction%20to%20affinity%20chromatography%26lt%3B%5C%2Fi%26gt%3B%20%283.%20printing%29.%20Elsevier%20Biomedical%20Press.%26lt%3B%5C%2Fdiv%26gt%3B%5Cn%26lt%3B%5C%2Fdiv%26gt%3B%22%2C%22data%22%3A%7B%22itemType%22%3A%22book%22%2C%22title%22%3A%22An%20introduction%20to%20affinity%20chromatography%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Christopher%20R.%22%2C%22lastName%22%3A%22Lowe%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22C.%20R.%22%2C%22lastName%22%3A%22Lowe%22%7D%5D%2C%22abstractNote%22%3A%22%22%2C%22date%22%3A%221985%22%2C%22originalDate%22%3A%22%22%2C%22originalPublisher%22%3A%22%22%2C%22originalPlace%22%3A%22%22%2C%22format%22%3A%22%22%2C%22ISBN%22%3A%229780720442236%22%2C%22DOI%22%3A%22%22%2C%22citationKey%22%3A%22%22%2C%22url%22%3A%22%22%2C%22ISSN%22%3A%22%22%2C%22language%22%3A%22eng%22%2C%22collections%22%3A%5B%22XAWSHPGP%22%5D%2C%22dateModified%22%3A%222025-12-23T18%3A45%3A14Z%22%7D%7D%2C%7B%22key%22%3A%22WZA8I8AK%22%2C%22library%22%3A%7B%22id%22%3A21495%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Rodriguez%20et%20al.%22%2C%22parsedDate%22%3A%222020%22%2C%22numChildren%22%3A0%7D%2C%22bib%22%3A%22%26lt%3Bdiv%20class%3D%26quot%3Bcsl-bib-body%26quot%3B%20style%3D%26quot%3Bline-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%26quot%3B%26gt%3B%5Cn%20%20%26lt%3Bdiv%20class%3D%26quot%3Bcsl-entry%26quot%3B%26gt%3BRodriguez%2C%20E.%20L.%2C%20Poddar%2C%20S.%2C%20Iftekhar%2C%20S.%2C%20Suh%2C%20K.%2C%20Woolfork%2C%20A.%20G.%2C%20Ovbude%2C%20S.%2C%20Pekarek%2C%20A.%2C%20Walters%2C%20M.%2C%20Lott%2C%20S.%2C%20%26amp%3B%20Hage%2C%20D.%20S.%20%282020%29.%20Affinity%20chromatography%3A%20A%20review%20of%20trends%20and%20developments%20over%20the%20past%2050%20years.%20%26lt%3Bi%26gt%3BJournal%20of%20Chromatography%20B%26lt%3B%5C%2Fi%26gt%3B%2C%20%26lt%3Bi%26gt%3B1157%26lt%3B%5C%2Fi%26gt%3B%2C%20122332.%20%26lt%3Ba%20class%3D%26%23039%3Bzp-DOIURL%26%23039%3B%20href%3D%26%23039%3Bhttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1016%5C%2Fj.jchromb.2020.122332%26%23039%3B%26gt%3Bhttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1016%5C%2Fj.jchromb.2020.122332%26lt%3B%5C%2Fa%26gt%3B%26lt%3B%5C%2Fdiv%26gt%3B%5Cn%26lt%3B%5C%2Fdiv%26gt%3B%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Affinity%20chromatography%3A%20A%20review%20of%20trends%20and%20developments%20over%20the%20past%2050%20years%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Elliott%20L.%22%2C%22lastName%22%3A%22Rodriguez%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Saumen%22%2C%22lastName%22%3A%22Poddar%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Sazia%22%2C%22lastName%22%3A%22Iftekhar%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Kyungah%22%2C%22lastName%22%3A%22Suh%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Ashley%20G.%22%2C%22lastName%22%3A%22Woolfork%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Susan%22%2C%22lastName%22%3A%22Ovbude%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Allegra%22%2C%22lastName%22%3A%22Pekarek%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Morgan%22%2C%22lastName%22%3A%22Walters%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Shae%22%2C%22lastName%22%3A%22Lott%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22David%20S.%22%2C%22lastName%22%3A%22Hage%22%7D%5D%2C%22abstractNote%22%3A%22%22%2C%22date%22%3A%2211%5C%2F2020%22%2C%22section%22%3A%22%22%2C%22partNumber%22%3A%22%22%2C%22partTitle%22%3A%22%22%2C%22DOI%22%3A%2210.1016%5C%2Fj.jchromb.2020.122332%22%2C%22citationKey%22%3A%22%22%2C%22url%22%3A%22https%3A%5C%2F%5C%2Flinkinghub.elsevier.com%5C%2Fretrieve%5C%2Fpii%5C%2FS1570023220309703%22%2C%22PMID%22%3A%22%22%2C%22PMCID%22%3A%22%22%2C%22ISSN%22%3A%2215700232%22%2C%22language%22%3A%22en%22%2C%22collections%22%3A%5B%22XAWSHPGP%22%5D%2C%22dateModified%22%3A%222025-12-22T22%3A21%3A35Z%22%7D%7D%2C%7B%22key%22%3A%22RT4XJVNM%22%2C%22library%22%3A%7B%22id%22%3A21495%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Burgess%22%2C%22parsedDate%22%3A%222018%22%2C%22numChildren%22%3A0%7D%2C%22bib%22%3A%22%26lt%3Bdiv%20class%3D%26quot%3Bcsl-bib-body%26quot%3B%20style%3D%26quot%3Bline-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%26quot%3B%26gt%3B%5Cn%20%20%26lt%3Bdiv%20class%3D%26quot%3Bcsl-entry%26quot%3B%26gt%3BBurgess%2C%20R.%20R.%20%282018%29.%20A%20brief%20practical%20review%20of%20size%20exclusion%20chromatography%3A%20Rules%20of%20thumb%2C%20limitations%2C%20and%20troubleshooting.%20%26lt%3Bi%26gt%3BProtein%20Expression%20and%20Purification%26lt%3B%5C%2Fi%26gt%3B%2C%20%26lt%3Bi%26gt%3B150%26lt%3B%5C%2Fi%26gt%3B%2C%2081%26%23x2013%3B85.%20%26lt%3Ba%20class%3D%26%23039%3Bzp-DOIURL%26%23039%3B%20href%3D%26%23039%3Bhttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1016%5C%2Fj.pep.2018.05.007%26%23039%3B%26gt%3Bhttps%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1016%5C%2Fj.pep.2018.05.007%26lt%3B%5C%2Fa%26gt%3B%26lt%3B%5C%2Fdiv%26gt%3B%5Cn%26lt%3B%5C%2Fdiv%26gt%3B%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22A%20brief%20practical%20review%20of%20size%20exclusion%20chromatography%3A%20Rules%20of%20thumb%2C%20limitations%2C%20and%20troubleshooting%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Richard%20R.%22%2C%22lastName%22%3A%22Burgess%22%7D%5D%2C%22abstractNote%22%3A%22%22%2C%22date%22%3A%2210%5C%2F2018%22%2C%22section%22%3A%22%22%2C%22partNumber%22%3A%22%22%2C%22partTitle%22%3A%22%22%2C%22DOI%22%3A%2210.1016%5C%2Fj.pep.2018.05.007%22%2C%22citationKey%22%3A%22%22%2C%22url%22%3A%22https%3A%5C%2F%5C%2Flinkinghub.elsevier.com%5C%2Fretrieve%5C%2Fpii%5C%2FS1046592818302092%22%2C%22PMID%22%3A%22%22%2C%22PMCID%22%3A%22%22%2C%22ISSN%22%3A%2210465928%22%2C%22language%22%3A%22en%22%2C%22collections%22%3A%5B%22XAWSHPGP%22%5D%2C%22dateModified%22%3A%222025-12-18T18%3A27%3A05Z%22%7D%7D%2C%7B%22key%22%3A%22JHQ8X4AB%22%2C%22library%22%3A%7B%22id%22%3A21495%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Garnett%20and%20Basset%22%2C%22parsedDate%22%3A%222005%22%2C%22numChildren%22%3A0%7D%2C%22bib%22%3A%22%26lt%3Bdiv%20class%3D%26quot%3Bcsl-bib-body%26quot%3B%20style%3D%26quot%3Bline-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%26quot%3B%26gt%3B%5Cn%20%20%26lt%3Bdiv%20class%3D%26quot%3Bcsl-entry%26quot%3B%26gt%3BGarnett%2C%20R.%20H.%20T.%2C%20%26amp%3B%20Basset%2C%20N.%20C.%20%282005%29.%20Placer%20Deposits.%20In%20%26lt%3Bi%26gt%3BEconomic%20Geology%20100th%20Anniversary%20Volume%26lt%3B%5C%2Fi%26gt%3B%20%28pp.%20813%26%23x2013%3B843%29.%20Society%20of%20Economic%20Geologists%20%28SEG%29.%26lt%3B%5C%2Fdiv%26gt%3B%5Cn%26lt%3B%5C%2Fdiv%26gt%3B%22%2C%22data%22%3A%7B%22itemType%22%3A%22bookSection%22%2C%22title%22%3A%22Placer%20Deposits%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22R.H.T.%22%2C%22lastName%22%3A%22Garnett%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22N.C.%22%2C%22lastName%22%3A%22Basset%22%7D%5D%2C%22abstractNote%22%3A%22%22%2C%22bookTitle%22%3A%22Economic%20Geology%20100th%20Anniversary%20Volume%22%2C%22date%22%3A%222005%22%2C%22originalDate%22%3A%22%22%2C%22originalPublisher%22%3A%22%22%2C%22originalPlace%22%3A%22%22%2C%22format%22%3A%22%22%2C%22ISBN%22%3A%22978-1-887483-01-8%22%2C%22DOI%22%3A%22%22%2C%22citationKey%22%3A%22%22%2C%22url%22%3A%22%22%2C%22ISSN%22%3A%22%22%2C%22language%22%3A%22%22%2C%22collections%22%3A%5B%5D%2C%22dateModified%22%3A%222025-12-17T20%3A47%3A27Z%22%7D%7D%2C%7B%22key%22%3A%2292BCBSEE%22%2C%22library%22%3A%7B%22id%22%3A21495%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Hedenquist%20and%20Society%20of%20Economic%20Geologists%22%2C%22parsedDate%22%3A%222005%22%2C%22numChildren%22%3A0%7D%2C%22bib%22%3A%22%26lt%3Bdiv%20class%3D%26quot%3Bcsl-bib-body%26quot%3B%20style%3D%26quot%3Bline-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%26quot%3B%26gt%3B%5Cn%20%20%26lt%3Bdiv%20class%3D%26quot%3Bcsl-entry%26quot%3B%26gt%3BHedenquist%2C%20J.%20W.%2C%20%26amp%3B%20Society%20of%20Economic%20Geologists%20%28Eds.%29.%20%282005%29.%20%26lt%3Bi%26gt%3BEconomic%20geology%3A%20one%20hundredth%20anniversary%20volume%3B%201905%20-%202005%26lt%3B%5C%2Fi%26gt%3B.%20Soc.%20of%20Economic%20Geologists.%26lt%3B%5C%2Fdiv%26gt%3B%5Cn%26lt%3B%5C%2Fdiv%26gt%3B%22%2C%22data%22%3A%7B%22itemType%22%3A%22book%22%2C%22title%22%3A%22Economic%20geology%3A%20one%20hundredth%20anniversary%20volume%3B%201905%20-%202005%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22editor%22%2C%22firstName%22%3A%22Jeffrey%20W.%22%2C%22lastName%22%3A%22Hedenquist%22%7D%2C%7B%22creatorType%22%3A%22editor%22%2C%22name%22%3A%22Society%20of%20Economic%20Geologists%22%7D%5D%2C%22abstractNote%22%3A%22%22%2C%22date%22%3A%222005%22%2C%22originalDate%22%3A%22%22%2C%22originalPublisher%22%3A%22%22%2C%22originalPlace%22%3A%22%22%2C%22format%22%3A%22%22%2C%22ISBN%22%3A%229781887483018%22%2C%22DOI%22%3A%22%22%2C%22citationKey%22%3A%22%22%2C%22url%22%3A%22%22%2C%22ISSN%22%3A%22%22%2C%22language%22%3A%22eng%22%2C%22collections%22%3A%5B%5D%2C%22dateModified%22%3A%222025-12-17T20%3A41%3A18Z%22%7D%7D%2C%7B%22key%22%3A%22F838WVTD%22%2C%22library%22%3A%7B%22id%22%3A21495%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Jahns%20and%20Burnham%22%2C%22parsedDate%22%3A%221969%22%2C%22numChildren%22%3A0%7D%2C%22bib%22%3A%22%26lt%3Bdiv%20class%3D%26quot%3Bcsl-bib-body%26quot%3B%20style%3D%26quot%3Bline-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%26quot%3B%26gt%3B%5Cn%20%20%26lt%3Bdiv%20class%3D%26quot%3Bcsl-entry%26quot%3B%26gt%3BJahns%2C%20R.%20H.%2C%20%26amp%3B%20Burnham%2C%20C.%20W.%20%281969%29.%20Experimental%20studies%20of%20pegmatite%20genesis%3A%20I.%20A%20model%20for%20the%20derivation%20and%20crystallization%20of%20granitic%20pegmatites.%20%26lt%3Bi%26gt%3BEconomic%20Geology%26lt%3B%5C%2Fi%26gt%3B%2C%20%26lt%3Bi%26gt%3B64%26lt%3B%5C%2Fi%26gt%3B%288%29%2C%20843%26%23x2013%3B864.%26lt%3B%5C%2Fdiv%26gt%3B%5Cn%26lt%3B%5C%2Fdiv%26gt%3B%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Experimental%20studies%20of%20pegmatite%20genesis%3A%20I.%20A%20model%20for%20the%20derivation%20and%20crystallization%20of%20granitic%20pegmatites%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22R.H.%22%2C%22lastName%22%3A%22Jahns%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22C.W.%22%2C%22lastName%22%3A%22Burnham%22%7D%5D%2C%22abstractNote%22%3A%22%22%2C%22date%22%3A%221969%22%2C%22section%22%3A%22%22%2C%22partNumber%22%3A%22%22%2C%22partTitle%22%3A%22%22%2C%22DOI%22%3A%22%22%2C%22citationKey%22%3A%22%22%2C%22url%22%3A%22%22%2C%22PMID%22%3A%22%22%2C%22PMCID%22%3A%22%22%2C%22ISSN%22%3A%22%22%2C%22language%22%3A%22%22%2C%22collections%22%3A%5B%5D%2C%22dateModified%22%3A%222025-12-17T20%3A19%3A29Z%22%7D%7D%2C%7B%22key%22%3A%2265RX37LN%22%2C%22library%22%3A%7B%22id%22%3A21495%7D%2C%22meta%22%3A%7B%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%26lt%3Bdiv%20class%3D%26quot%3Bcsl-bib-body%26quot%3B%20style%3D%26quot%3Bline-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%26quot%3B%26gt%3B%5Cn%20%20%26lt%3Bdiv%20class%3D%26quot%3Bcsl-entry%26quot%3B%26gt%3BIgneous%20Rocks.%20%28n.d.%29.%20%26lt%3Bi%26gt%3BEarth%40Home%26lt%3B%5C%2Fi%26gt%3B.%20Retrieved%20December%2017%2C%202025%2C%20from%20%26lt%3Ba%20class%3D%26%23039%3Bzp-ItemURL%26%23039%3B%20href%3D%26%23039%3Bhttps%3A%5C%2F%5C%2Fearthathome.org%5C%2Fde%5C%2Frocks%5C%2Figneous%5C%2F%26%23039%3B%26gt%3Bhttps%3A%5C%2F%5C%2Fearthathome.org%5C%2Fde%5C%2Frocks%5C%2Figneous%5C%2F%26lt%3B%5C%2Fa%26gt%3B%26lt%3B%5C%2Fdiv%26gt%3B%5Cn%26lt%3B%5C%2Fdiv%26gt%3B%22%2C%22data%22%3A%7B%22itemType%22%3A%22blogPost%22%2C%22title%22%3A%22Igneous%20Rocks%22%2C%22creators%22%3A%5B%5D%2C%22abstractNote%22%3A%22Overview%20Igneous%20rocks%20form%20from%20the%20cooling%20of%20magma%20%28molten%20rock%20underground%29%20or%20lava%20%28molten%20rock%20at%20the%20Earth%26%23039%3Bs%20surface%29.%20There%20are%20two%20major%20types%20of%20igneous%20rocks%3A%20plutonic%20and%20volcanic.%20These%20two%20major%20groups%20are%20differentiated%20based%20on%20whether%20they%20cooled%20above%20or%20below%20Earth%26%23039%3Bs%20surface%2C%20which%20in%20turn%20dictates%20whether%20or%20not%20crystals%20are%20visible%20to%20the%20naked%20...%20Read%20More%22%2C%22blogTitle%22%3A%22Earth%40Home%22%2C%22date%22%3A%22%22%2C%22DOI%22%3A%22%22%2C%22citationKey%22%3A%22%22%2C%22url%22%3A%22https%3A%5C%2F%5C%2Fearthathome.org%5C%2Fde%5C%2Frocks%5C%2Figneous%5C%2F%22%2C%22ISSN%22%3A%22%22%2C%22language%22%3A%22en-US%22%2C%22collections%22%3A%5B%5D%2C%22dateModified%22%3A%222025-12-17T19%3A30%3A34Z%22%7D%7D%2C%7B%22key%22%3A%22Y2482AAT%22%2C%22library%22%3A%7B%22id%22%3A21495%7D%2C%22meta%22%3A%7B%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%26lt%3Bdiv%20class%3D%26quot%3Bcsl-bib-body%26quot%3B%20style%3D%26quot%3Bline-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%26quot%3B%26gt%3B%5Cn%20%20%26lt%3Bdiv%20class%3D%26quot%3Bcsl-entry%26quot%3B%26gt%3B%26lt%3Bi%26gt%3BPractising%20science%3A%20Reading%20the%20rocks%20and%20ecology%26lt%3B%5C%2Fi%26gt%3B.%20%28n.d.%29.%20Open%20Learning.%20Retrieved%20December%2017%2C%202025%2C%20from%20%26lt%3Ba%20class%3D%26%23039%3Bzp-ItemURL%26%23039%3B%20href%3D%26%23039%3Bhttps%3A%5C%2F%5C%2Fwww.open.edu%5C%2Fopenlearn%5C%2Fscience-maths-technology%5C%2Fgeology%5C%2Fpractising-science-reading-the-rocks-and-ecology%5C%2Fcontent-section-1.3.2%26%23039%3B%26gt%3Bhttps%3A%5C%2F%5C%2Fwww.open.edu%5C%2Fopenlearn%5C%2Fscience-maths-technology%5C%2Fgeology%5C%2Fpractising-science-reading-the-rocks-and-ecology%5C%2Fcontent-section-1.3.2%26lt%3B%5C%2Fa%26gt%3B%26lt%3B%5C%2Fdiv%26gt%3B%5Cn%26lt%3B%5C%2Fdiv%26gt%3B%22%2C%22data%22%3A%7B%22itemType%22%3A%22webpage%22%2C%22title%22%3A%22Practising%20science%3A%20Reading%20the%20rocks%20and%20ecology%22%2C%22creators%22%3A%5B%5D%2C%22abstractNote%22%3A%22Have%20you%20ever%20wondered%20how%20scientists%20analyse%20the%20environment%3F%20This%20free%20course%2C%20Practising%20science%3A%20Reading%20the%20rocks%20and%20ecology%2C%20introduces%20you%20to%20the%20techniques%20used%20by%20science%20students%20at%20...%22%2C%22date%22%3A%22%22%2C%22DOI%22%3A%22%22%2C%22citationKey%22%3A%22%22%2C%22url%22%3A%22https%3A%5C%2F%5C%2Fwww.open.edu%5C%2Fopenlearn%5C%2Fscience-maths-technology%5C%2Fgeology%5C%2Fpractising-science-reading-the-rocks-and-ecology%5C%2Fcontent-section-1.3.2%22%2C%22language%22%3A%22en%22%2C%22collections%22%3A%5B%5D%2C%22dateModified%22%3A%222025-12-17T19%3A00%3A39Z%22%7D%7D%2C%7B%22key%22%3A%22W7BST8MD%22%2C%22library%22%3A%7B%22id%22%3A21495%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Michael%20E.%20Ritter%22%2C%22parsedDate%22%3A%222021-10-09%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%26lt%3Bdiv%20class%3D%26quot%3Bcsl-bib-body%26quot%3B%20style%3D%26quot%3Bline-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%26quot%3B%26gt%3B%5Cn%20%20%26lt%3Bdiv%20class%3D%26quot%3Bcsl-entry%26quot%3B%26gt%3BMichael%20E.%20Ritter.%20%282021%2C%20October%209%29.%20%26lt%3Bi%26gt%3B14.5.2%3A%20Igneous%20Rocks%26lt%3B%5C%2Fi%26gt%3B.%20Geosciences%20LibreTexts.%20%26lt%3Ba%20class%3D%26%23039%3Bzp-ItemURL%26%23039%3B%20href%3D%26%23039%3Bhttps%3A%5C%2F%5C%2Fgeo.libretexts.org%5C%2FBookshelves%5C%2FGeography_%28Physical%29%5C%2FThe_Physical_Environment_%28Ritter%29%5C%2F14%253A_Earth_Materials_and_Structure%5C%2F14.05%253A_Rocks%5C%2F14.5.02%253A_Igneous_Rocks%26%23039%3B%26gt%3Bhttps%3A%5C%2F%5C%2Fgeo.libretexts.org%5C%2FBookshelves%5C%2FGeography_%28Physical%29%5C%2FThe_Physical_Environment_%28Ritter%29%5C%2F14%253A_Earth_Materials_and_Structure%5C%2F14.05%253A_Rocks%5C%2F14.5.02%253A_Igneous_Rocks%26lt%3B%5C%2Fa%26gt%3B%26lt%3B%5C%2Fdiv%26gt%3B%5Cn%26lt%3B%5C%2Fdiv%26gt%3B%22%2C%22data%22%3A%7B%22itemType%22%3A%22webpage%22%2C%22title%22%3A%2214.5.2%3A%20Igneous%20Rocks%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22name%22%3A%22Michael%20E.%20Ritter%22%7D%5D%2C%22abstractNote%22%3A%22%22%2C%22date%22%3A%222021-10-09T23%3A55%3A43Z%22%2C%22DOI%22%3A%22%22%2C%22citationKey%22%3A%22%22%2C%22url%22%3A%22https%3A%5C%2F%5C%2Fgeo.libretexts.org%5C%2FBookshelves%5C%2FGeography_%28Physical%29%5C%2FThe_Physical_Environment_%28Ritter%29%5C%2F14%253A_Earth_Materials_and_Structure%5C%2F14.05%253A_Rocks%5C%2F14.5.02%253A_Igneous_Rocks%22%2C%22language%22%3A%22en%22%2C%22collections%22%3A%5B%5D%2C%22dateModified%22%3A%222025-12-17T18%3A58%3A15Z%22%7D%7D%2C%7B%22key%22%3A%22WDXSI6GS%22%2C%22library%22%3A%7B%22id%22%3A21495%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22James%20E.%20Shigley%20and%20Aaron%20C.%20Palke%22%2C%22parsedDate%22%3A%222022%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%26lt%3Bdiv%20class%3D%26quot%3Bcsl-bib-body%26quot%3B%20style%3D%26quot%3Bline-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%26quot%3B%26gt%3B%5Cn%20%20%26lt%3Bdiv%20class%3D%26quot%3Bcsl-entry%26quot%3B%26gt%3BJames%20E.%20Shigley%2C%20%26amp%3B%20Aaron%20C.%20Palke.%20%282022%29.%20Gems%20Formed%20in%20Magmatic%20Rocks.%20%26lt%3Bi%26gt%3BGems%20%26amp%3B%20Gemology%26lt%3B%5C%2Fi%26gt%3B%2C%20%26lt%3Bi%26gt%3B58%26lt%3B%5C%2Fi%26gt%3B%284%29%2C%20494%26%23x2013%3B506.%20%26lt%3Ba%20class%3D%26%23039%3Bzp-ItemURL%26%23039%3B%20href%3D%26%23039%3Bhttp%3A%5C%2F%5C%2Fwww.gia.edu%5C%2Fsites%5C%2FSatellite%3Fc%3DPage%26amp%3Bcid%3D1495338607530%26amp%3Bchildpagename%3DGIA%5C%2FPage%5C%2FGGArticleDetail%26amp%3Bpagename%3DGIA%5C%2FWrapper%26amp%3BWRAPPERPAGE%3DGIA%5C%2FWrapper%26%23039%3B%26gt%3Bhttp%3A%5C%2F%5C%2Fwww.gia.edu%5C%2Fsites%5C%2FSatellite%3Fc%3DPage%26amp%3Bcid%3D1495338607530%26amp%3Bchildpagename%3DGIA%5C%2FPage%5C%2FGGArticleDetail%26amp%3Bpagename%3DGIA%5C%2FWrapper%26amp%3BWRAPPERPAGE%3DGIA%5C%2FWrapper%26lt%3B%5C%2Fa%26gt%3B%26lt%3B%5C%2Fdiv%26gt%3B%5Cn%26lt%3B%5C%2Fdiv%26gt%3B%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%22Gems%20Formed%20in%20Magmatic%20Rocks%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22name%22%3A%22James%20E.%20Shigley%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22name%22%3A%22Aaron%20C.%20Palke%22%7D%5D%2C%22abstractNote%22%3A%22This%20installment%20of%20%5Cu201cColored%20Stones%20Unearthed%5Cu201d%20covers%20gemstones%20that%20formed%20in%20magmatic%20environments%20and%20the%20insights%20they%20offer%20into%20the%20evolution%20of%20the%20earth%20and%20the%20geological%20processes%20that%20shaped%20our%20world.%22%2C%22date%22%3A%222022%22%2C%22section%22%3A%22%22%2C%22partNumber%22%3A%22%22%2C%22partTitle%22%3A%22%22%2C%22DOI%22%3A%22%22%2C%22citationKey%22%3A%22%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Fwww.gia.edu%5C%2Fsites%5C%2FSatellite%3Fc%3DPage%26cid%3D1495338607530%26childpagename%3DGIA%5C%2FPage%5C%2FGGArticleDetail%26pagename%3DGIA%5C%2FWrapper%26WRAPPERPAGE%3DGIA%5C%2FWrapper%22%2C%22PMID%22%3A%22%22%2C%22PMCID%22%3A%22%22%2C%22ISSN%22%3A%22%22%2C%22language%22%3A%22en%22%2C%22collections%22%3A%5B%5D%2C%22dateModified%22%3A%222025-12-17T18%3A55%3A31Z%22%7D%7D%2C%7B%22key%22%3A%22WECJZ94P%22%2C%22library%22%3A%7B%22id%22%3A21495%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Panchuk%22%2C%22parsedDate%22%3A%222021-08-20%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%26lt%3Bdiv%20class%3D%26quot%3Bcsl-bib-body%26quot%3B%20style%3D%26quot%3Bline-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%26quot%3B%26gt%3B%5Cn%20%20%26lt%3Bdiv%20class%3D%26quot%3Bcsl-entry%26quot%3B%26gt%3BPanchuk%2C%20K.%20%282021%29.%20%26lt%3Bi%26gt%3B7.2%20Crystallization%20of%20Magma%26lt%3B%5C%2Fi%26gt%3B.%20%26lt%3Ba%20class%3D%26%23039%3Bzp-ItemURL%26%23039%3B%20href%3D%26%23039%3Bhttps%3A%5C%2F%5C%2Fopentextbc.ca%5C%2Fphysicalgeologyh5p%5C%2Fchapter%5C%2Fcrystallization-of-magma%5C%2F%26%23039%3B%26gt%3Bhttps%3A%5C%2F%5C%2Fopentextbc.ca%5C%2Fphysicalgeologyh5p%5C%2Fchapter%5C%2Fcrystallization-of-magma%5C%2F%26lt%3B%5C%2Fa%26gt%3B%26lt%3B%5C%2Fdiv%26gt%3B%5Cn%26lt%3B%5C%2Fdiv%26gt%3B%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%227.2%20Crystallization%20of%20Magma%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Karla%22%2C%22lastName%22%3A%22Panchuk%22%7D%5D%2C%22abstractNote%22%3A%22%26lt%3Bi%26gt%3BPhysical%20Geology%20-%20H5P%20Edition%26lt%3B%5C%2Fi%26gt%3B%20is%20an%20interactive%20comprehensive%20introductory%20text%20on%20the%20physical%20aspects%20of%20geology%2C%20including%20rocks%20and%20minerals%2C%20plate%20tectonics%2C%20earthquakes%2C%20volcanoes%2C%20mass%20wasting%2C%20climate%20change%2C%20planetary%20geology%2C%20and%20more.%20It%20has%20a%20strong%20emphasis%20on%20examples%20from%20western%20Canada.%22%2C%22date%22%3A%222021-08-20%22%2C%22section%22%3A%22%22%2C%22partNumber%22%3A%22%22%2C%22partTitle%22%3A%22%22%2C%22DOI%22%3A%22%22%2C%22citationKey%22%3A%22%22%2C%22url%22%3A%22https%3A%5C%2F%5C%2Fopentextbc.ca%5C%2Fphysicalgeologyh5p%5C%2Fchapter%5C%2Fcrystallization-of-magma%5C%2F%22%2C%22PMID%22%3A%22%22%2C%22PMCID%22%3A%22%22%2C%22ISSN%22%3A%22%22%2C%22language%22%3A%22en%22%2C%22collections%22%3A%5B%5D%2C%22dateModified%22%3A%222025-12-17T18%3A27%3A00Z%22%7D%7D%2C%7B%22key%22%3A%227CRGKQYU%22%2C%22library%22%3A%7B%22id%22%3A21495%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Solada%20and%20Daniels%22%2C%22parsedDate%22%3A%222021%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%26lt%3Bdiv%20class%3D%26quot%3Bcsl-bib-body%26quot%3B%20style%3D%26quot%3Bline-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%26quot%3B%26gt%3B%5Cn%20%20%26lt%3Bdiv%20class%3D%26quot%3Bcsl-entry%26quot%3B%26gt%3BSolada%2C%20K.%2C%20%26amp%3B%20Daniels%2C%20K.%20S.%20%282021%29.%20%26lt%3Bi%26gt%3B4.2%20Crystallization%20of%20Magma%26lt%3B%5C%2Fi%26gt%3B.%20%26lt%3Ba%20class%3D%26%23039%3Bzp-ItemURL%26%23039%3B%20href%3D%26%23039%3Bhttps%3A%5C%2F%5C%2Fopenoregon.pressbooks.pub%5C%2Fearthscience%5C%2Fchapter%5C%2F4-2-crystallization-of-magma-2%5C%2F%26%23039%3B%26gt%3Bhttps%3A%5C%2F%5C%2Fopenoregon.pressbooks.pub%5C%2Fearthscience%5C%2Fchapter%5C%2F4-2-crystallization-of-magma-2%5C%2F%26lt%3B%5C%2Fa%26gt%3B%26lt%3B%5C%2Fdiv%26gt%3B%5Cn%26lt%3B%5C%2Fdiv%26gt%3B%22%2C%22data%22%3A%7B%22itemType%22%3A%22journalArticle%22%2C%22title%22%3A%224.2%20Crystallization%20of%20Magma%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Katharine%22%2C%22lastName%22%3A%22Solada%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22K.%20Sean%22%2C%22lastName%22%3A%22Daniels%22%7D%5D%2C%22abstractNote%22%3A%22This%20book%20focuses%20on%20Earth%20Science%20for%20entry-level%20or%20non-science%20majors.%22%2C%22date%22%3A%222021%22%2C%22section%22%3A%22%22%2C%22partNumber%22%3A%22%22%2C%22partTitle%22%3A%22%22%2C%22DOI%22%3A%22%22%2C%22citationKey%22%3A%22%22%2C%22url%22%3A%22https%3A%5C%2F%5C%2Fopenoregon.pressbooks.pub%5C%2Fearthscience%5C%2Fchapter%5C%2F4-2-crystallization-of-magma-2%5C%2F%22%2C%22PMID%22%3A%22%22%2C%22PMCID%22%3A%22%22%2C%22ISSN%22%3A%22%22%2C%22language%22%3A%22en%22%2C%22collections%22%3A%5B%5D%2C%22dateModified%22%3A%222025-12-17T18%3A22%3A24Z%22%7D%7D%2C%7B%22key%22%3A%22UYD7YYGK%22%2C%22library%22%3A%7B%22id%22%3A21495%7D%2C%22meta%22%3A%7B%22numChildren%22%3A0%7D%2C%22bib%22%3A%22%26lt%3Bdiv%20class%3D%26quot%3Bcsl-bib-body%26quot%3B%20style%3D%26quot%3Bline-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%26quot%3B%26gt%3B%5Cn%20%20%26lt%3Bdiv%20class%3D%26quot%3Bcsl-entry%26quot%3B%26gt%3B%26lt%3Bi%26gt%3BMagma%20crystallization%20%7C%20Research%20Starters%20%7C%20EBSCO%20Research%26lt%3B%5C%2Fi%26gt%3B.%20%28n.d.%29.%20EBSCO.%20Retrieved%20December%2017%2C%202025%2C%20from%20%26lt%3Ba%20class%3D%26%23039%3Bzp-ItemURL%26%23039%3B%20href%3D%26%23039%3Bhttps%3A%5C%2F%5C%2Fwww.ebsco.com%26%23039%3B%26gt%3Bhttps%3A%5C%2F%5C%2Fwww.ebsco.com%26lt%3B%5C%2Fa%26gt%3B%26lt%3B%5C%2Fdiv%26gt%3B%5Cn%26lt%3B%5C%2Fdiv%26gt%3B%22%2C%22data%22%3A%7B%22itemType%22%3A%22webpage%22%2C%22title%22%3A%22Magma%20crystallization%20%7C%20Research%20Starters%20%7C%20EBSCO%20Research%22%2C%22creators%22%3A%5B%5D%2C%22abstractNote%22%3A%22%26lt%3Bp%26gt%3BMagma%20crystallization%20is%20the%20process%20by%20which%20molten%20rock%2C%20known%20as%20magma%2C%20cools%20and%20solidifies%20to%20form%20igneous%20rock.%20This%20process%20typically%20occurs%20either%20deep%20within%20the%20Earth%5Cu2019s%20crust%20or%20at%20the%20surface%20when%20magma%20erupts%20as%20lava.%20As%20magma%20cools%2C%20minerals%20crystallize%20in%20a%20specific%20sequence%20based%20on%20temperature%2C%20beginning%20with%20those%20rich%20in%20calcium%2C%20iron%2C%20and%20magnesium%2C%20and%20progressing%20to%20those%20with%20higher%20concentrations%20of%20aluminum%2C%20potassium%2C%20and%20sodium.%20Slow%20cooling%20beneath%20the%20surface%20allows%20for%20larger%20crystal%20growth%2C%20resulting%20in%20rocks%20with%20a%20phaneritic%20texture%2C%20while%20rapid%20cooling%20at%20the%20surface%20produces%20fine-grained%20or%20glassy%20rocks%2C%20characterized%20as%20aphanitic.%26lt%3B%5C%2Fp%26gt%3B%5Cn%26lt%3Bp%26gt%3BCrystallization%20not%20only%20leads%20to%20the%20formation%20of%20rocks%20but%20also%20influences%20the%20concentration%20of%20valuable%20elements.%20For%20instance%2C%20certain%20minerals%2C%20like%20diamonds%2C%20originate%20from%20deep%20within%20the%20mantle%20and%20are%20transported%20to%20the%20surface%20by%20specific%20types%20of%20magma%20called%20kimberlite.%20Additionally%2C%20magma%20can%20form%20layered%20intrusions%20where%20minerals%20are%20organized%20in%20a%20repeating%20sequence%20due%20to%20gravity%20layering%2C%20contributing%20to%20major%20deposits%20of%20metals%20such%20as%20platinum%20and%20chromium.%20Overall%2C%20magma%20crystallization%20is%20a%20fundamental%20geological%20process%20that%20plays%20a%20crucial%20role%20in%20the%20formation%20of%20the%20Earth%5Cu2019s%20crust%20and%20the%20availability%20of%20various%20mineral%20resources.%26lt%3B%5C%2Fp%26gt%3B%22%2C%22date%22%3A%22%22%2C%22DOI%22%3A%22%22%2C%22citationKey%22%3A%22%22%2C%22url%22%3A%22https%3A%5C%2F%5C%2Fwww.ebsco.com%22%2C%22language%22%3A%22en%22%2C%22collections%22%3A%5B%5D%2C%22dateModified%22%3A%222025-12-17T18%3A22%3A05Z%22%7D%7D%2C%7B%22key%22%3A%2232DN797A%22%2C%22library%22%3A%7B%22id%22%3A21495%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Bowen%22%2C%22parsedDate%22%3A%221928%22%2C%22numChildren%22%3A0%7D%2C%22bib%22%3A%22%26lt%3Bdiv%20class%3D%26quot%3Bcsl-bib-body%26quot%3B%20style%3D%26quot%3Bline-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%26quot%3B%26gt%3B%5Cn%20%20%26lt%3Bdiv%20class%3D%26quot%3Bcsl-entry%26quot%3B%26gt%3BBowen%2C%20N.%20L.%20%281928%29.%20%26lt%3Bi%26gt%3BThe%20evolution%20of%20the%20igneous%20rocks%26lt%3B%5C%2Fi%26gt%3B.%20Princeton%2C%20Princeton%20University%20Press.%20%26lt%3Ba%20class%3D%26%23039%3Bzp-ItemURL%26%23039%3B%20href%3D%26%23039%3Bhttp%3A%5C%2F%5C%2Farchive.org%5C%2Fdetails%5C%2Fevolutionofigneo00bowe%26%23039%3B%26gt%3Bhttp%3A%5C%2F%5C%2Farchive.org%5C%2Fdetails%5C%2Fevolutionofigneo00bowe%26lt%3B%5C%2Fa%26gt%3B%26lt%3B%5C%2Fdiv%26gt%3B%5Cn%26lt%3B%5C%2Fdiv%26gt%3B%22%2C%22data%22%3A%7B%22itemType%22%3A%22book%22%2C%22title%22%3A%22The%20evolution%20of%20the%20igneous%20rocks%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Norman%20Levi%22%2C%22lastName%22%3A%22Bowen%22%7D%2C%7B%22creatorType%22%3A%22contributor%22%2C%22name%22%3A%22Internet%20Archive%22%7D%5D%2C%22abstractNote%22%3A%22%26quot%3BBased%20on%20a%20brief%20course%20of%20lectures%20given%20to%20advanced%20students%20in%20the%20Department%20of%20geology%20at%20Princeton%20in%20the%20spring%20of%201927.%26quot%3B--Pref%3B%20%26quot%3BSelected%20bibliography%26quot%3B%20at%20end%20of%20chapter%20IX%3B%20bibliographical%20footnotes%22%2C%22date%22%3A%221928%22%2C%22originalDate%22%3A%22%22%2C%22originalPublisher%22%3A%22%22%2C%22originalPlace%22%3A%22%22%2C%22format%22%3A%22%22%2C%22ISBN%22%3A%22%22%2C%22DOI%22%3A%22%22%2C%22citationKey%22%3A%22%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Farchive.org%5C%2Fdetails%5C%2Fevolutionofigneo00bowe%22%2C%22ISSN%22%3A%22%22%2C%22language%22%3A%22eng%22%2C%22collections%22%3A%5B%5D%2C%22dateModified%22%3A%222025-12-17T18%3A02%3A20Z%22%7D%7D%2C%7B%22key%22%3A%22N9TSSWGP%22%2C%22library%22%3A%7B%22id%22%3A21495%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Waseem%22%2C%22parsedDate%22%3A%222025-09-12%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%26lt%3Bdiv%20class%3D%26quot%3Bcsl-bib-body%26quot%3B%20style%3D%26quot%3Bline-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%26quot%3B%26gt%3B%5Cn%20%20%26lt%3Bdiv%20class%3D%26quot%3Bcsl-entry%26quot%3B%26gt%3BWaseem%2C%20U.%20%282025%2C%20September%2012%29.%20%26lt%3Bi%26gt%3BCrystalline%20vs%20Amorphous%3A%20Structure%2C%20Properties%20and%20Engineering%20Implications%26lt%3B%5C%2Fi%26gt%3B.%20Wevolver.%20%26lt%3Ba%20class%3D%26%23039%3Bzp-ItemURL%26%23039%3B%20href%3D%26%23039%3Bhttps%3A%5C%2F%5C%2Fwww.wevolver.com%5C%2Farticle%5C%2Fcrystalline-vs-amorphous-structure-properties-and-engineering-implications%26%23039%3B%26gt%3Bhttps%3A%5C%2F%5C%2Fwww.wevolver.com%5C%2Farticle%5C%2Fcrystalline-vs-amorphous-structure-properties-and-engineering-implications%26lt%3B%5C%2Fa%26gt%3B%26lt%3B%5C%2Fdiv%26gt%3B%5Cn%26lt%3B%5C%2Fdiv%26gt%3B%22%2C%22data%22%3A%7B%22itemType%22%3A%22webpage%22%2C%22title%22%3A%22Crystalline%20vs%20Amorphous%3A%20Structure%2C%20Properties%20and%20Engineering%20Implications%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Umar%22%2C%22lastName%22%3A%22Waseem%22%7D%5D%2C%22abstractNote%22%3A%22This%20article%20is%20a%20detailed%20exploration%20of%20crystalline%20vs%20amorphous%20solids%2C%20covering%20atomic%20order%2C%20materials%20properties%2C%20semiconductors%2C%20and%20how%20they%20translate%20into%20practical%20semiconductor%2C%20hardware%20and%20digital%20design%20applications.%22%2C%22date%22%3A%22Fri%20Sep%2012%202025%2005%3A15%3A28%20GMT-0400%20%28Eastern%20Daylight%20Time%29%22%2C%22DOI%22%3A%22%22%2C%22citationKey%22%3A%22%22%2C%22url%22%3A%22https%3A%5C%2F%5C%2Fwww.wevolver.com%5C%2Farticle%5C%2Fcrystalline-vs-amorphous-structure-properties-and-engineering-implications%22%2C%22language%22%3A%22%22%2C%22collections%22%3A%5B%5D%2C%22dateModified%22%3A%222025-12-15T17%3A48%3A13Z%22%7D%7D%2C%7B%22key%22%3A%22R3KUEC4E%22%2C%22library%22%3A%7B%22id%22%3A21495%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Grace%20Conyers%22%2C%22parsedDate%22%3A%222019-02-25%22%2C%22numChildren%22%3A0%7D%2C%22bib%22%3A%22%26lt%3Bdiv%20class%3D%26quot%3Bcsl-bib-body%26quot%3B%20style%3D%26quot%3Bline-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%26quot%3B%26gt%3B%5Cn%20%20%26lt%3Bdiv%20class%3D%26quot%3Bcsl-entry%26quot%3B%26gt%3BGrace%20Conyers.%20%282019%2C%20February%2025%29.%20Make%20Bismuth%20Crystals.%20%26lt%3Bi%26gt%3BInsanitek%26lt%3B%5C%2Fi%26gt%3B.%20%26lt%3Ba%20class%3D%26%23039%3Bzp-ItemURL%26%23039%3B%20href%3D%26%23039%3Bhttps%3A%5C%2F%5C%2Finsanitek.net%5C%2Fmake-bismuth-crystals%5C%2F%26%23039%3B%26gt%3Bhttps%3A%5C%2F%5C%2Finsanitek.net%5C%2Fmake-bismuth-crystals%5C%2F%26lt%3B%5C%2Fa%26gt%3B%26lt%3B%5C%2Fdiv%26gt%3B%5Cn%26lt%3B%5C%2Fdiv%26gt%3B%22%2C%22data%22%3A%7B%22itemType%22%3A%22blogPost%22%2C%22title%22%3A%22Make%20Bismuth%20Crystals%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22name%22%3A%22Grace%20Conyers%22%7D%5D%2C%22abstractNote%22%3A%22Bismuth%20crystals.%20They%20are%20majestic%20and%20beautiful%20looking%20structures%2C%20and%20since%20bismuth%20melts%20at%20520%5Cu00b0F%2C%20it%20can%20even%20be%20made%20at%20home%22%2C%22blogTitle%22%3A%22Insanitek%22%2C%22date%22%3A%222019-02-25T18%3A01%3A58%2B00%3A00%22%2C%22DOI%22%3A%22%22%2C%22citationKey%22%3A%22%22%2C%22url%22%3A%22https%3A%5C%2F%5C%2Finsanitek.net%5C%2Fmake-bismuth-crystals%5C%2F%22%2C%22ISSN%22%3A%22%22%2C%22language%22%3A%22en-US%22%2C%22collections%22%3A%5B%5D%2C%22dateModified%22%3A%222025-12-15T16%3A41%3A12Z%22%7D%7D%2C%7B%22key%22%3A%22JP9RQ8VA%22%2C%22library%22%3A%7B%22id%22%3A21495%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Mettler-Toledo%20International%20Inc%20all%20rights%22%2C%22numChildren%22%3A0%7D%2C%22bib%22%3A%22%26lt%3Bdiv%20class%3D%26quot%3Bcsl-bib-body%26quot%3B%20style%3D%26quot%3Bline-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%26quot%3B%26gt%3B%5Cn%20%20%26lt%3Bdiv%20class%3D%26quot%3Bcsl-entry%26quot%3B%26gt%3BMettler-Toledo%20International%20Inc%20all%20rights.%20%28n.d.%29.%20%26lt%3Bi%26gt%3BSupersaturation%20and%20Crystallization%26lt%3B%5C%2Fi%26gt%3B.%20Retrieved%20December%2015%2C%202025%2C%20from%20%26lt%3Ba%20class%3D%26%23039%3Bzp-ItemURL%26%23039%3B%20href%3D%26%23039%3Bhttps%3A%5C%2F%5C%2Fwww.mt.com%5C%2Fus%5C%2Fen%5C%2Fhome%5C%2Fapplications%5C%2FL1_AutoChem_Applications%5C%2FL2_Crystallization%5C%2FSupersaturation_Application.html%26%23039%3B%26gt%3Bhttps%3A%5C%2F%5C%2Fwww.mt.com%5C%2Fus%5C%2Fen%5C%2Fhome%5C%2Fapplications%5C%2FL1_AutoChem_Applications%5C%2FL2_Crystallization%5C%2FSupersaturation_Application.html%26lt%3B%5C%2Fa%26gt%3B%26lt%3B%5C%2Fdiv%26gt%3B%5Cn%26lt%3B%5C%2Fdiv%26gt%3B%22%2C%22data%22%3A%7B%22itemType%22%3A%22webpage%22%2C%22title%22%3A%22Supersaturation%20and%20Crystallization%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22name%22%3A%22Mettler-Toledo%20International%20Inc%20all%20rights%22%7D%5D%2C%22abstractNote%22%3A%22Supersaturation%20occurs%20when%20a%20solution%20contains%20more%20solute%20than%20should%20be%20possible%20thermodynamically%2C%20given%20the%20conditions%20of%20the%20system.%20Supersaturation%20is%20considered%20a%20major%20driver%20for%20cryst...%22%2C%22date%22%3A%22%22%2C%22DOI%22%3A%22%22%2C%22citationKey%22%3A%22%22%2C%22url%22%3A%22https%3A%5C%2F%5C%2Fwww.mt.com%5C%2Fus%5C%2Fen%5C%2Fhome%5C%2Fapplications%5C%2FL1_AutoChem_Applications%5C%2FL2_Crystallization%5C%2FSupersaturation_Application.html%22%2C%22language%22%3A%22en-US%22%2C%22collections%22%3A%5B%5D%2C%22dateModified%22%3A%222025-12-15T16%3A37%3A01Z%22%7D%7D%2C%7B%22key%22%3A%227Y7NH3E7%22%2C%22library%22%3A%7B%22id%22%3A21495%7D%2C%22meta%22%3A%7B%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%26lt%3Bdiv%20class%3D%26quot%3Bcsl-bib-body%26quot%3B%20style%3D%26quot%3Bline-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%26quot%3B%26gt%3B%5Cn%20%20%26lt%3Bdiv%20class%3D%26quot%3Bcsl-entry%26quot%3B%26gt%3B%26lt%3Bi%26gt%3BGrowing%20Quality%20Crystals%20%26%23x2013%3B%20MIT%20Department%20of%20Chemistry%26lt%3B%5C%2Fi%26gt%3B.%20%28n.d.%29.%20Retrieved%20December%2015%2C%202025%2C%20from%20%26lt%3Ba%20class%3D%26%23039%3Bzp-ItemURL%26%23039%3B%20href%3D%26%23039%3Bhttps%3A%5C%2F%5C%2Fchemistry.mit.edu%5C%2Ffacilities-and-centers%5C%2Fx-ray-diffraction-facility%5C%2Fgrowing-quality-crystals%5C%2F%26%23039%3B%26gt%3Bhttps%3A%5C%2F%5C%2Fchemistry.mit.edu%5C%2Ffacilities-and-centers%5C%2Fx-ray-diffraction-facility%5C%2Fgrowing-quality-crystals%5C%2F%26lt%3B%5C%2Fa%26gt%3B%26lt%3B%5C%2Fdiv%26gt%3B%5Cn%26lt%3B%5C%2Fdiv%26gt%3B%22%2C%22data%22%3A%7B%22itemType%22%3A%22blogPost%22%2C%22title%22%3A%22Growing%20Quality%20Crystals%20%5Cu2013%20MIT%20Department%20of%20Chemistry%22%2C%22creators%22%3A%5B%5D%2C%22abstractNote%22%3A%22%22%2C%22blogTitle%22%3A%22%22%2C%22date%22%3A%22%22%2C%22DOI%22%3A%22%22%2C%22citationKey%22%3A%22%22%2C%22url%22%3A%22https%3A%5C%2F%5C%2Fchemistry.mit.edu%5C%2Ffacilities-and-centers%5C%2Fx-ray-diffraction-facility%5C%2Fgrowing-quality-crystals%5C%2F%22%2C%22ISSN%22%3A%22%22%2C%22language%22%3A%22en-US%22%2C%22collections%22%3A%5B%5D%2C%22dateModified%22%3A%222025-12-15T16%3A17%3A04Z%22%7D%7D%2C%7B%22key%22%3A%2238NKMJ7J%22%2C%22library%22%3A%7B%22id%22%3A21495%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Kujan%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%26lt%3Bdiv%20class%3D%26quot%3Bcsl-bib-body%26quot%3B%20style%3D%26quot%3Bline-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%26quot%3B%26gt%3B%5Cn%20%20%26lt%3Bdiv%20class%3D%26quot%3Bcsl-entry%26quot%3B%26gt%3BKujan%2C%20S.%20L.%20%28n.d.%29.%20%26lt%3Bi%26gt%3BCrystallization%20from%20a%20Supersaturated%20Solution%26lt%3B%5C%2Fi%26gt%3B.%20Department%20of%20Chemistry%20%26amp%3B%20Chemical%20Biology%20%7C%20Rutgers%2C%20The%20State%20University%20of%20New%20Jersey.%20Retrieved%20December%2015%2C%202025%2C%20from%20%26lt%3Ba%20class%3D%26%23039%3Bzp-ItemURL%26%23039%3B%20href%3D%26%23039%3Bhttps%3A%5C%2F%5C%2Fchem.rutgers.edu%5C%2Fcldf-demos%5C%2F1031-cldf-demo-crystallization%26%23039%3B%26gt%3Bhttps%3A%5C%2F%5C%2Fchem.rutgers.edu%5C%2Fcldf-demos%5C%2F1031-cldf-demo-crystallization%26lt%3B%5C%2Fa%26gt%3B%26lt%3B%5C%2Fdiv%26gt%3B%5Cn%26lt%3B%5C%2Fdiv%26gt%3B%22%2C%22data%22%3A%7B%22itemType%22%3A%22webpage%22%2C%22title%22%3A%22Crystallization%20from%20a%20Supersaturated%20Solution%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Stephen%20L.%22%2C%22lastName%22%3A%22Kujan%22%7D%5D%2C%22abstractNote%22%3A%22Department%20of%20Chemistry%20%26amp%3B%20Chemical%20Biology%2C%20The%20School%20of%20Arts%20and%20Sciences%2C%20Rutgers%2C%20The%20State%20University%20of%20New%20Jersey%22%2C%22date%22%3A%22%22%2C%22DOI%22%3A%22%22%2C%22citationKey%22%3A%22%22%2C%22url%22%3A%22https%3A%5C%2F%5C%2Fchem.rutgers.edu%5C%2Fcldf-demos%5C%2F1031-cldf-demo-crystallization%22%2C%22language%22%3A%22en-gb%22%2C%22collections%22%3A%5B%5D%2C%22dateModified%22%3A%222025-12-15T16%3A16%3A56Z%22%7D%7D%2C%7B%22key%22%3A%223ALRDRLU%22%2C%22library%22%3A%7B%22id%22%3A21495%7D%2C%22meta%22%3A%7B%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%26lt%3Bdiv%20class%3D%26quot%3Bcsl-bib-body%26quot%3B%20style%3D%26quot%3Bline-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%26quot%3B%26gt%3B%5Cn%20%20%26lt%3Bdiv%20class%3D%26quot%3Bcsl-entry%26quot%3B%26gt%3B%26lt%3Bi%26gt%3BSupersaturation%20and%20Crystallization%20%7C%20Harvard%20Natural%20Sciences%20Lecture%20Demonstrations%26lt%3B%5C%2Fi%26gt%3B.%20%28n.d.%29.%20Retrieved%20December%2015%2C%202025%2C%20from%20%26lt%3Ba%20class%3D%26%23039%3Bzp-ItemURL%26%23039%3B%20href%3D%26%23039%3Bhttps%3A%5C%2F%5C%2Fsciencedemonstrations.fas.harvard.edu%5C%2Fpresentations%5C%2Fsupersaturation-and-crystallization%26%23039%3B%26gt%3Bhttps%3A%5C%2F%5C%2Fsciencedemonstrations.fas.harvard.edu%5C%2Fpresentations%5C%2Fsupersaturation-and-crystallization%26lt%3B%5C%2Fa%26gt%3B%26lt%3B%5C%2Fdiv%26gt%3B%5Cn%26lt%3B%5C%2Fdiv%26gt%3B%22%2C%22data%22%3A%7B%22itemType%22%3A%22webpage%22%2C%22title%22%3A%22Supersaturation%20and%20Crystallization%20%7C%20Harvard%20Natural%20Sciences%20Lecture%20Demonstrations%22%2C%22creators%22%3A%5B%5D%2C%22abstractNote%22%3A%22What%20it%20shows%3A%20A%20supersaturated%20solution%20is%20unstable%2C%20and%20by%20seeding%20it%20you%20can%20trigger%20rapid%20crystallization.How%20it%20works%3A%20Sodium%20acetate%20can%20dissolve%20in%20water%20in%20great%22%2C%22date%22%3A%22%22%2C%22DOI%22%3A%22%22%2C%22citationKey%22%3A%22%22%2C%22url%22%3A%22https%3A%5C%2F%5C%2Fsciencedemonstrations.fas.harvard.edu%5C%2Fpresentations%5C%2Fsupersaturation-and-crystallization%22%2C%22language%22%3A%22en%22%2C%22collections%22%3A%5B%5D%2C%22dateModified%22%3A%222025-12-15T16%3A08%3A47Z%22%7D%7D%2C%7B%22key%22%3A%228R9EVEKG%22%2C%22library%22%3A%7B%22id%22%3A21495%7D%2C%22meta%22%3A%7B%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%26lt%3Bdiv%20class%3D%26quot%3Bcsl-bib-body%26quot%3B%20style%3D%26quot%3Bline-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%26quot%3B%26gt%3B%5Cn%20%20%26lt%3Bdiv%20class%3D%26quot%3Bcsl-entry%26quot%3B%26gt%3B%26lt%3Bi%26gt%3BIon%20Exchange%20Chromatography%26lt%3B%5C%2Fi%26gt%3B.%20%28n.d.%29.%20Retrieved%20December%2011%2C%202025%2C%20from%20%26lt%3Ba%20class%3D%26%23039%3Bzp-ItemURL%26%23039%3B%20href%3D%26%23039%3Bhttps%3A%5C%2F%5C%2Fwww.shimadzu.com%5C%2Fan%5C%2Fservice-support%5C%2Ftechnical-support%5C%2Fanalysis-basics%5C%2Fbasic%5C%2Fion_exchange_chromatography.html%26%23039%3B%26gt%3Bhttps%3A%5C%2F%5C%2Fwww.shimadzu.com%5C%2Fan%5C%2Fservice-support%5C%2Ftechnical-support%5C%2Fanalysis-basics%5C%2Fbasic%5C%2Fion_exchange_chromatography.html%26lt%3B%5C%2Fa%26gt%3B%26lt%3B%5C%2Fdiv%26gt%3B%5Cn%26lt%3B%5C%2Fdiv%26gt%3B%22%2C%22data%22%3A%7B%22itemType%22%3A%22webpage%22%2C%22title%22%3A%22Ion%20Exchange%20Chromatography%22%2C%22creators%22%3A%5B%5D%2C%22abstractNote%22%3A%22%22%2C%22date%22%3A%22%22%2C%22DOI%22%3A%22%22%2C%22citationKey%22%3A%22%22%2C%22url%22%3A%22https%3A%5C%2F%5C%2Fwww.shimadzu.com%5C%2Fan%5C%2Fservice-support%5C%2Ftechnical-support%5C%2Fanalysis-basics%5C%2Fbasic%5C%2Fion_exchange_chromatography.html%22%2C%22language%22%3A%22en%22%2C%22collections%22%3A%5B%5D%2C%22dateModified%22%3A%222025-12-11T14%3A27%3A58Z%22%7D%7D%2C%7B%22key%22%3A%22D9T2CF3F%22%2C%22library%22%3A%7B%22id%22%3A21495%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Curling%22%2C%22parsedDate%22%3A%222025-12-10%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%26lt%3Bdiv%20class%3D%26quot%3Bcsl-bib-body%26quot%3B%20style%3D%26quot%3Bline-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%26quot%3B%26gt%3B%5Cn%20%20%26lt%3Bdiv%20class%3D%26quot%3Bcsl-entry%26quot%3B%26gt%3BCurling%2C%20J.%20%282025%2C%20December%2010%29.%20%26lt%3Bi%26gt%3BHISTORY%20OF%20CHROMATOGRAPHY%3A%20Process%20Chromatography%3A%20Five%20Decades%20of%20Innovation%20%7C%20BioPharm%20International%26lt%3B%5C%2Fi%26gt%3B.%20%26lt%3Ba%20class%3D%26%23039%3Bzp-ItemURL%26%23039%3B%20href%3D%26%23039%3Bhttps%3A%5C%2F%5C%2Fwww.biopharminternational.com%5C%2Fview%5C%2Fhistory-chromatography-process-chromatography-five-decades-innovation%26%23039%3B%26gt%3Bhttps%3A%5C%2F%5C%2Fwww.biopharminternational.com%5C%2Fview%5C%2Fhistory-chromatography-process-chromatography-five-decades-innovation%26lt%3B%5C%2Fa%26gt%3B%26lt%3B%5C%2Fdiv%26gt%3B%5Cn%26lt%3B%5C%2Fdiv%26gt%3B%22%2C%22data%22%3A%7B%22itemType%22%3A%22webpage%22%2C%22title%22%3A%22HISTORY%20OF%20CHROMATOGRAPHY%3A%20Process%20Chromatography%3A%20Five%20Decades%20of%20Innovation%20%7C%20BioPharm%20International%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22John%22%2C%22lastName%22%3A%22Curling%22%7D%5D%2C%22abstractNote%22%3A%22This%20article%20explores%20the%20development%20of%20process%20chromatography.%20Process%20chromatography%20was%20first%20applied%20to%20the%20removal%20of%20low%20molecular%20weight%20solutes%20from%20whey%20by%20gel%20filtration%20about%2050%20years%20ago.%20An%20analytical%20method%20using%20size%20exclusion%20chromatography%20was%20scaled%20up%20for%20insulin%20production%20in%20the%201970s%2C%20when%20ion%20exchange%20became%20a%20viable%20technology%20for%20the%20same%20application.%20Ion%20exchange%20was%20adopted%20as%20the%20industry%20workhorse%20as%20robust%20resins%20became%20available%20and%20formed%20the%20backbone%20of%20chromatographic%20processing%20of%20blood%20plasma%20fractionation%20in%20alternatives%20to%20and%20extensions%20of%20ethanol%20precipitation.%22%2C%22date%22%3A%222025-12-10T19%3A48%3A34.662Z%22%2C%22DOI%22%3A%22%22%2C%22citationKey%22%3A%22%22%2C%22url%22%3A%22https%3A%5C%2F%5C%2Fwww.biopharminternational.com%5C%2Fview%5C%2Fhistory-chromatography-process-chromatography-five-decades-innovation%22%2C%22language%22%3A%22en%22%2C%22collections%22%3A%5B%22XAWSHPGP%22%5D%2C%22dateModified%22%3A%222025-12-10T19%3A48%3A41Z%22%7D%7D%2C%7B%22key%22%3A%22GX47WVAD%22%2C%22library%22%3A%7B%22id%22%3A21495%7D%2C%22meta%22%3A%7B%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%26lt%3Bdiv%20class%3D%26quot%3Bcsl-bib-body%26quot%3B%20style%3D%26quot%3Bline-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%26quot%3B%26gt%3B%5Cn%20%20%26lt%3Bdiv%20class%3D%26quot%3Bcsl-entry%26quot%3B%26gt%3B%26lt%3Bi%26gt%3BIon%20Exchange%5CnChromatography%3A%20Principles%20and%20Methods%26lt%3B%5C%2Fi%26gt%3B.%20%28n.d.%29.%20GE%20Healthcare.%26lt%3B%5C%2Fdiv%26gt%3B%5Cn%26lt%3B%5C%2Fdiv%26gt%3B%22%2C%22data%22%3A%7B%22itemType%22%3A%22book%22%2C%22title%22%3A%22Ion%20Exchange%5CnChromatography%3A%20Principles%20and%20Methods%22%2C%22creators%22%3A%5B%5D%2C%22abstractNote%22%3A%22%22%2C%22date%22%3A%22%22%2C%22originalDate%22%3A%22%22%2C%22originalPublisher%22%3A%22%22%2C%22originalPlace%22%3A%22%22%2C%22format%22%3A%22%22%2C%22ISBN%22%3A%22%22%2C%22DOI%22%3A%22%22%2C%22citationKey%22%3A%22%22%2C%22url%22%3A%22%22%2C%22ISSN%22%3A%22%22%2C%22language%22%3A%22%22%2C%22collections%22%3A%5B%22XAWSHPGP%22%5D%2C%22dateModified%22%3A%222025-12-10T19%3A27%3A52Z%22%7D%7D%2C%7B%22key%22%3A%222NQZCYVH%22%2C%22library%22%3A%7B%22id%22%3A21495%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Jungbauer%20and%20Hahn%22%2C%22parsedDate%22%3A%222009%22%2C%22numChildren%22%3A0%7D%2C%22bib%22%3A%22%26lt%3Bdiv%20class%3D%26quot%3Bcsl-bib-body%26quot%3B%20style%3D%26quot%3Bline-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%26quot%3B%26gt%3B%5Cn%20%20%26lt%3Bdiv%20class%3D%26quot%3Bcsl-entry%26quot%3B%26gt%3BJungbauer%2C%20A.%2C%20%26amp%3B%20Hahn%2C%20R.%20%282009%29.%20Chapter%2022%20Ion-Exchange%20Chromatography.%20In%20%26lt%3Bi%26gt%3BMethods%20in%20Enzymology%26lt%3B%5C%2Fi%26gt%3B%20%28Vol.%20463%2C%20pp.%20349%26%23x2013%3B371%29.%20Elsevier.%20https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1016%5C%2FS0076-6879%2809%2963022-6%26lt%3B%5C%2Fdiv%26gt%3B%5Cn%26lt%3B%5C%2Fdiv%26gt%3B%22%2C%22data%22%3A%7B%22itemType%22%3A%22bookSection%22%2C%22title%22%3A%22Chapter%2022%20Ion-Exchange%20Chromatography%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Alois%22%2C%22lastName%22%3A%22Jungbauer%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Rainer%22%2C%22lastName%22%3A%22Hahn%22%7D%5D%2C%22abstractNote%22%3A%22%22%2C%22bookTitle%22%3A%22Methods%20in%20Enzymology%22%2C%22date%22%3A%222009%22%2C%22originalDate%22%3A%22%22%2C%22originalPublisher%22%3A%22%22%2C%22originalPlace%22%3A%22%22%2C%22format%22%3A%22%22%2C%22ISBN%22%3A%229780123745361%22%2C%22DOI%22%3A%22%22%2C%22citationKey%22%3A%22%22%2C%22url%22%3A%22https%3A%5C%2F%5C%2Flinkinghub.elsevier.com%5C%2Fretrieve%5C%2Fpii%5C%2FS0076687909630226%22%2C%22ISSN%22%3A%22%22%2C%22language%22%3A%22en%22%2C%22collections%22%3A%5B%22XAWSHPGP%22%5D%2C%22dateModified%22%3A%222025-12-10T19%3A16%3A44Z%22%7D%7D%2C%7B%22key%22%3A%22QSRSJUEW%22%2C%22library%22%3A%7B%22id%22%3A21495%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22McSweeny%22%2C%22parsedDate%22%3A%222025-10-17%22%2C%22numChildren%22%3A0%7D%2C%22bib%22%3A%22%26lt%3Bdiv%20class%3D%26quot%3Bcsl-bib-body%26quot%3B%20style%3D%26quot%3Bline-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%26quot%3B%26gt%3B%5Cn%20%20%26lt%3Bdiv%20class%3D%26quot%3Bcsl-entry%26quot%3B%26gt%3BMcSweeny%2C%20E.%20%282025%2C%20October%2017%29.%20All%20Charged%20Up%3A%20The%20Basics%20of%20Ion-Exchange%20Chromatography%20%5BScience%20Lab%20Support%5D.%20%26lt%3Bi%26gt%3BBiteSized%20Bio%26lt%3B%5C%2Fi%26gt%3B.%20%26lt%3Ba%20class%3D%26%23039%3Bzp-ItemURL%26%23039%3B%20href%3D%26%23039%3Bhttps%3A%5C%2F%5C%2Fbitesizebio.com%5C%2F31744%5C%2Fbasics-ion-exchange-chromatography%5C%2F%26%23039%3B%26gt%3Bhttps%3A%5C%2F%5C%2Fbitesizebio.com%5C%2F31744%5C%2Fbasics-ion-exchange-chromatography%5C%2F%26lt%3B%5C%2Fa%26gt%3B%26lt%3B%5C%2Fdiv%26gt%3B%5Cn%26lt%3B%5C%2Fdiv%26gt%3B%22%2C%22data%22%3A%7B%22itemType%22%3A%22blogPost%22%2C%22title%22%3A%22All%20Charged%20Up%3A%20The%20Basics%20of%20Ion-Exchange%20Chromatography%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Elizabeth%22%2C%22lastName%22%3A%22McSweeny%22%7D%5D%2C%22abstractNote%22%3A%22%22%2C%22blogTitle%22%3A%22BiteSized%20Bio%22%2C%22date%22%3A%222025-10-17%22%2C%22DOI%22%3A%22%22%2C%22citationKey%22%3A%22%22%2C%22url%22%3A%22https%3A%5C%2F%5C%2Fbitesizebio.com%5C%2F31744%5C%2Fbasics-ion-exchange-chromatography%5C%2F%22%2C%22ISSN%22%3A%22%22%2C%22language%22%3A%22%22%2C%22collections%22%3A%5B%22XAWSHPGP%22%5D%2C%22dateModified%22%3A%222025-12-10T19%3A04%3A56Z%22%7D%7D%2C%7B%22key%22%3A%2239EW2GJM%22%2C%22library%22%3A%7B%22id%22%3A21495%7D%2C%22meta%22%3A%7B%22parsedDate%22%3A%222021-10-15%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%26lt%3Bdiv%20class%3D%26quot%3Bcsl-bib-body%26quot%3B%20style%3D%26quot%3Bline-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%26quot%3B%26gt%3B%5Cn%20%20%26lt%3Bdiv%20class%3D%26quot%3Bcsl-entry%26quot%3B%26gt%3B%26lt%3Bi%26gt%3BIon-Exchange%20Chromatography%3A%20An%20Easy%20Introduction%20to%20the%20Basics%26lt%3B%5C%2Fi%26gt%3B.%20%282021%2C%20October%2015%29.%20%26lt%3Ba%20class%3D%26%23039%3Bzp-ItemURL%26%23039%3B%20href%3D%26%23039%3Bhttps%3A%5C%2F%5C%2Fbitesizebio.com%5C%2F31744%5C%2Fbasics-ion-exchange-chromatography%5C%2F%26%23039%3B%26gt%3Bhttps%3A%5C%2F%5C%2Fbitesizebio.com%5C%2F31744%5C%2Fbasics-ion-exchange-chromatography%5C%2F%26lt%3B%5C%2Fa%26gt%3B%26lt%3B%5C%2Fdiv%26gt%3B%5Cn%26lt%3B%5C%2Fdiv%26gt%3B%22%2C%22data%22%3A%7B%22itemType%22%3A%22webpage%22%2C%22title%22%3A%22Ion-Exchange%20Chromatography%3A%20An%20Easy%20Introduction%20to%20the%20Basics%22%2C%22creators%22%3A%5B%5D%2C%22abstractNote%22%3A%22Ion-exchange%20chromatography%20is%20used%20to%20separate%20and%20purify%20proteins%20based%20on%20net%20charge%20at%20a%20particular%20pH.%20Here%20are%20the%20basics%20about%20this%20technique.%22%2C%22date%22%3A%222021-10-15T09%3A00%3A00%2B01%3A00%22%2C%22DOI%22%3A%22%22%2C%22citationKey%22%3A%22%22%2C%22url%22%3A%22https%3A%5C%2F%5C%2Fbitesizebio.com%5C%2F31744%5C%2Fbasics-ion-exchange-chromatography%5C%2F%22%2C%22language%22%3A%22en-US%22%2C%22collections%22%3A%5B%5D%2C%22dateModified%22%3A%222025-12-10T19%3A00%3A37Z%22%7D%7D%2C%7B%22key%22%3A%22G4PSMRBP%22%2C%22library%22%3A%7B%22id%22%3A21495%7D%2C%22meta%22%3A%7B%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%26lt%3Bdiv%20class%3D%26quot%3Bcsl-bib-body%26quot%3B%20style%3D%26quot%3Bline-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%26quot%3B%26gt%3B%5Cn%20%20%26lt%3Bdiv%20class%3D%26quot%3Bcsl-entry%26quot%3B%26gt%3B%26lt%3Bi%26gt%3BThe%202%20Common%20Types%20of%20Ion%20Exchange%20Chromatography%3A%20Anion%20vs.%20Cation%26lt%3B%5C%2Fi%26gt%3B.%20%28n.d.%29.%20GoldBio.%20Retrieved%20December%2010%2C%202025%2C%20from%20%26lt%3Ba%20class%3D%26%23039%3Bzp-ItemURL%26%23039%3B%20href%3D%26%23039%3Bhttps%3A%5C%2F%5C%2Fwww.goldbio.com%5C%2Fblogs%5C%2Farticles%5C%2F2-common-types-of-ion-exchange-chromatography-anion-vs-cation%26%23039%3B%26gt%3Bhttps%3A%5C%2F%5C%2Fwww.goldbio.com%5C%2Fblogs%5C%2Farticles%5C%2F2-common-types-of-ion-exchange-chromatography-anion-vs-cation%26lt%3B%5C%2Fa%26gt%3B%26lt%3B%5C%2Fdiv%26gt%3B%5Cn%26lt%3B%5C%2Fdiv%26gt%3B%22%2C%22data%22%3A%7B%22itemType%22%3A%22webpage%22%2C%22title%22%3A%22The%202%20Common%20Types%20of%20Ion%20Exchange%20Chromatography%3A%20Anion%20vs.%20Cation%22%2C%22creators%22%3A%5B%5D%2C%22abstractNote%22%3A%22Ion%20exchange%20chromatography%20includes%20a%20suite%20of%20related%20methods%20that%20purify%20proteins%20based%20on%20their%20charge.%5CnThe%20two%20main%20types%20of%20ion%20exchange%20resins%20are%20anion%20and%20cation%2C%20which%20bind%20to%20negatively%20and%20positively%20charged%20molecules%2C%20respectively.%20There%20are%20several%20functional%20groups%20for%20anion%20and%20cation%20exchange%20chromatography%2C%20as%20well%20as%20different%20resolutions.%20%5CnChoosing%20the%20appropriate%20resin%20and%20resolution%20type%20will%20depend%20on%20your%20protein%20of%20interest%20and%20purification%20purpose.%5CnIf%20you%20know%20that%20you%20need%20to%20do%20ion%20exchange%20chromatography%20to%20purify%20your%20protein%2C%20but%20you%5Cu2019re%20not%20sure%20exactly%20what%20type%20of%20ion%20exchange%20you%20should%20do%20%5Cu2013%20you%5Cu2019re%20in%20the%20right%20place%21%20Follow%20along%20as%20we%20discuss%20the%20different%20types%20of%20resins%20used%20for%20ion%20exchange.%5Cn%5CnArticle%20Contents%3A%5Cn%5CnCation%20Exchange%20vs%20Anion%20Exchange%5CnBuffer%20pH%20in%20Ion%20Exchange%20Chromatography%5CnCation%20Exchange%5CnAnion%20Exchange%5CnResolution%5CnRelated%20Products%5CnReferences%5Cn%5Cn%20%5CnCation%20Exchange%20vs%20Anion%20Exchange%5CnThere%20are%20two%20main%20types%20of%20ion%20exchange%3A%20cation%20exchange%20and%20anion%20exchange%20chromatography.%20Let%5Cu2019s%20go%20through%20exactly%20what%20these%20are%2C%20because%20the%20nomenclature%20can%20seem%20counterintuitive%20at%20first.%5CnA%20cation-exchange%20resin%20is%20actually%20negatively%20charged%20and%20binds%20positively-charged%20proteins.%20Conversely%2C%20anion-exchange%20resins%20are%20positively%20charged%20and%20bind%20negatively-charged%20proteins%20%28Figure%201%29.%5Cn%5CnFigure%201.%20Cation%20exchange%20resin%20has%20negatively%20charged%20agarose%20beads%20that%20bind%20positively%20charged%20proteins%20%28left%29.%20Anion%20exchange%20resin%20has%20positively%20charged%20agarose%20beads%20that%20bind%20negatively%20charged%20proteins%20%28right%29.%5CnSo%20the%20first%20thing%20to%20figure%20out%20is%20the%20charge%20of%20the%20protein%20that%20you%20are%20trying%20to%20purify.%5Cn%5Cn%5Cn%5Cn%5Cn%5CnBuffer%20pH%20in%20Ion%20Exchange%20Chromatography%5Cn%5CnA%20protein%5Cu2019s%20charge%2C%20however%2C%20depends%20on%20the%20pH%20of%20the%20buffer%20or%20the%20environment%20that%20it%20is%20in.%20There%20are%20a%20lot%20of%20important%20features%20of%20protein%20purification%20buffers%2C%20and%20we%20discuss%20many%20of%20those%20features%20in%20depth%20here.%20For%20ion%20exchange%20chromatography%20buffers%20in%20particular%2C%20buffer%20pH%20and%20salt%20concentration%20are%20the%20two%20important%20variables%20that%20we%20will%20focus%20on%20in%20this%20article.%5CnTo%20determine%20whether%20your%20protein%20will%20be%20positive%20or%20negative%20at%20a%20given%20pH%2C%20you%20need%20to%20calculate%20the%20isoelectric%20point%2C%20conventionally%20denoted%20as%20%5Cu201cpI%2C%5Cu201d%20of%20a%20protein.%20You%20can%20check%20out%20this%20article%20to%20learn%20how%20to%20estimate%20a%20protein%5Cu2019s%20pI%2C%20and%20how%20to%20use%20that%20information%20to%20determine%20which%20pH%20will%20be%20good%20for%20purifying%20your%20protein%20via%20ion%20exchange.%5Cn%5CnCation%20Exchange%5CnThere%20are%20many%20different%20cation%20exchange%20resins.%20They%20all%20bind%20positively%20charged%20molecules%2C%20but%20they%20use%20slightly%20different%20functional%20groups%20to%20do%20so.%20See%20Table%201%20for%20a%20list%20of%20commonly%20used%20cation-exchange%20resins.%5CnTable%201.%20Cation%20exchange%20functional%20groups.%5Cn%5Cn%5CnOne%20distinction%20you%20will%20see%20in%20Table%201%20is%20strong%20vs.%20weak%20cation%20exchangers.%20Strong%20indicates%20that%20a%20chemical%20moiety%20remains%20charged%2C%20and%20therefore%20useful%20for%20binding%20cations%2C%20across%20a%20broad%20pH%20range%20whereas%20weak%20exchangers%20are%20useful%20over%20a%20more%20limited%20pH%20range.%5CnS%20columns%20are%20strong%20exchangers%20and%20are%20frequently%20used%20as%20a%20standard%20default%20choice%20for%20cation%20exchange.%20However%2C%20if%20you%20are%20working%20off%20of%20an%20existing%20protocol%20that%20calls%20for%20another%20type%20of%20cation%20exchange%20resin%2C%20you%20will%20probably%20want%20to%20try%20that%20type%20of%20resin%20first.%5CnHeparin%20columns%20combine%20features%20of%20ion%20exchange%20%28IEX%29%20columns%20and%20affinity%20columns%20in%20that%20they%20are%20especially%20good%20at%20binding%20positively-charged%20proteins%20that%20bind%20to%20polysaccharides%20or%20nucleic%20acids%20%28Siri%20et%20al%2C%201986%29%2C%20so%20they%20are%20a%20good%20choice%20for%20these%20types%20of%20proteins.%5Cn%5Cn%5CnAnion%20Exchange%5CnSimilarly%2C%20there%20are%20many%20different%20types%20of%20anion%20exchange%20resins%20that%20bind%20negatively%20charged%20molecules%20using%20different%20functional%20groups.%20See%20Table%202%20for%20a%20list%20of%20frequently%20used%20anion%20exchange%20resins.%5Cn%20Table%202.%20Anion%20exchange%20functional%20groups.%20%5Cn%5CnJust%20like%20cation%20exchangers%2C%20strong%20anion%20exchangers%20are%20charged%20over%20a%20broad%20pH%20range%20whereas%20weak%20exchangers%20work%20over%20a%20more%20limited%20pH%20range.%5CnQ%20columns%20are%20strong%20anion%20exchangers%20and%20are%20frequently%20used%20as%20the%20default%20choice%2C%20so%20if%20you%5Cu2019re%20not%20sure%20what%20to%20use%2C%20start%20there.%20However%2C%20if%20you%5Cu2019re%20working%20off%20an%20existing%20protocol%20that%20uses%20a%20different%20anion%20exchange%20resin%2C%20you%20should%20try%20the%20same%20one%20first.%5CnDEAE%20columns%20are%20particularly%20good%20at%20binding%20to%20nucleic%20acids%20and%20are%20frequently%20used%20to%20purify%20DNA%20and%20RNA%20away%20from%20a%20protein%20during%20a%20purification%20%28Ali%20et%20al%2C%202017%29.%5Cn%5CnResolution%5CnWhether%20using%20cation%20or%20anion%20exchange%20resins%2C%20you%20will%20also%20need%20to%20select%20whether%20to%20use%20a%20resin%20that%20is%20high%20or%20moderate%20resolution.%20Resolution%20refers%20to%20the%20ability%20of%20the%20column%20to%20separate%20out%20different%20proteins%20that%20have%20similar%20charge%20properties%20%28Figure%202%29.%5Cn%5Cn%20Figure%202.%20Resolution%20differences%20between%20columns%20determine%20whether%20proteins%20%28different%20purple%20shapes%29%20coelute%20%28left%29%20or%20are%20separated%20%28right%29%20during%20an%20IEX%20purification.%20%5Cn%5CnFor%20ion%20exchange%20chromatography%2C%20resolution%20is%20primarily%20a%20function%20of%20resin%20bead%20size.%20The%20smaller%20the%20beads%2C%20the%20higher%20the%20resolution%20whereas%20the%20larger%20the%20beads%20the%20lower%20the%20resolution%20%28Figure%203%29.%5CnThere%20is%20also%20a%20tradeoff%20between%20resolution%20and%20speed%20in%20that%20high-resolution%20resins%20are%20run%20at%20a%20lower%20flow%20rate%20and%20will%20therefore%20take%20longer.%5CnOften%20the%20decision%20on%20which%20type%20of%20resolution%20is%20optimal%20depends%20on%20the%20exact%20purpose%20you%20are%20using%20ion%20exchange%20for.%20When%20it%20is%20an%20additional%20purification%20step%20between%20affinity%20tag%20and%20size%20exclusion%20purifications%2C%20it%20is%20probably%20fine%20to%20use%20moderate%20resolution%20ion%20exchange%20chromatography%20and%20enjoy%20the%20faster%20purification.%5CnHowever%2C%20there%20are%20some%20special%20uses%20of%20ion%20exchange%20purification%20steps%20where%20it%20may%20be%20worth%20using%20high-resolution%20resin%20to%20purify%20proteins%20with%20posttranslational%20modifications%20or%20of%20different%20oligomeric%20states.%5Cn%5Cn%5CnFigure%203.%20Smaller%20bead%20size%20%28gray%20circles%29%20leads%20to%20increased%20purification%20resolution%20in%20ion%20exchange%20chromatography.%5Cn%5CnSo%20now%20you%20know%20a%20little%20bit%20more%20about%20the%20different%20ion%20exchange%20resin%20types%20and%20how%20to%20pick%20which%20resin%20and%20which%20resolution%20you%20want%20to%20try.%22%2C%22date%22%3A%22%22%2C%22DOI%22%3A%22%22%2C%22citationKey%22%3A%22%22%2C%22url%22%3A%22https%3A%5C%2F%5C%2Fwww.goldbio.com%5C%2Fblogs%5C%2Farticles%5C%2F2-common-types-of-ion-exchange-chromatography-anion-vs-cation%22%2C%22language%22%3A%22en%22%2C%22collections%22%3A%5B%5D%2C%22dateModified%22%3A%222025-12-10T18%3A53%3A40Z%22%7D%7D%2C%7B%22key%22%3A%22FZ35292N%22%2C%22library%22%3A%7B%22id%22%3A21495%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Kevin%20Ahern%20and%20Indira%20Rajagopal%22%2C%22parsedDate%22%3A%222019-07-15%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%26lt%3Bdiv%20class%3D%26quot%3Bcsl-bib-body%26quot%3B%20style%3D%26quot%3Bline-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%26quot%3B%26gt%3B%5Cn%20%20%26lt%3Bdiv%20class%3D%26quot%3Bcsl-entry%26quot%3B%26gt%3BKevin%20Ahern%2C%20%26amp%3B%20Indira%20Rajagopal.%20%282019%2C%20July%2015%29.%20%26lt%3Bi%26gt%3B3.4.3.%20Ion%20Exchange%20Chromatography%26lt%3B%5C%2Fi%26gt%3B.%20Chemistry%20LibreTexts.%20%26lt%3Ba%20class%3D%26%23039%3Bzp-ItemURL%26%23039%3B%20href%3D%26%23039%3Bhttps%3A%5C%2F%5C%2Fchem.libretexts.org%5C%2FCourses%5C%2FUniversity_of_Arkansas_Little_Rock%5C%2FCHEM_4320_5320%253A_Biochemistry_1%5C%2F03%253A_Methods_of_Protein_Purification_and_Characterization%5C%2F3.4%253A_Chromatography%5C%2F3.4.3._Ion_Exchange_Chromatography%26%23039%3B%26gt%3Bhttps%3A%5C%2F%5C%2Fchem.libretexts.org%5C%2FCourses%5C%2FUniversity_of_Arkansas_Little_Rock%5C%2FCHEM_4320_5320%253A_Biochemistry_1%5C%2F03%253A_Methods_of_Protein_Purification_and_Characterization%5C%2F3.4%253A_Chromatography%5C%2F3.4.3._Ion_Exchange_Chromatography%26lt%3B%5C%2Fa%26gt%3B%26lt%3B%5C%2Fdiv%26gt%3B%5Cn%26lt%3B%5C%2Fdiv%26gt%3B%22%2C%22data%22%3A%7B%22itemType%22%3A%22webpage%22%2C%22title%22%3A%223.4.3.%20Ion%20Exchange%20Chromatography%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22name%22%3A%22Kevin%20Ahern%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22name%22%3A%22Indira%20Rajagopal%22%7D%5D%2C%22abstractNote%22%3A%22Proteins%20can%20be%20separated%20on%20the%20basis%20of%20their%20net%20charge%20by%20ion-exchange%20chromatography.%20If%20a%20protein%20has%20a%20net%20positive%20charge%20at%20pH%207%2C%20it%20will%20bind%20to%20a%20column%20of%20beads%20containing%20carboxylate%20%5Cu2026%22%2C%22date%22%3A%222019-07-15T05%3A50%3A33Z%22%2C%22DOI%22%3A%22%22%2C%22citationKey%22%3A%22%22%2C%22url%22%3A%22https%3A%5C%2F%5C%2Fchem.libretexts.org%5C%2FCourses%5C%2FUniversity_of_Arkansas_Little_Rock%5C%2FCHEM_4320_5320%253A_Biochemistry_1%5C%2F03%253A_Methods_of_Protein_Purification_and_Characterization%5C%2F3.4%253A_Chromatography%5C%2F3.4.3._Ion_Exchange_Chromatography%22%2C%22language%22%3A%22en%22%2C%22collections%22%3A%5B%22XAWSHPGP%22%5D%2C%22dateModified%22%3A%222025-12-10T18%3A48%3A32Z%22%7D%7D%2C%7B%22key%22%3A%223AQDI8AH%22%2C%22library%22%3A%7B%22id%22%3A21495%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Small%22%2C%22parsedDate%22%3A%222025-12-10%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%26lt%3Bdiv%20class%3D%26quot%3Bcsl-bib-body%26quot%3B%20style%3D%26quot%3Bline-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%26quot%3B%26gt%3B%5Cn%20%20%26lt%3Bdiv%20class%3D%26quot%3Bcsl-entry%26quot%3B%26gt%3BSmall%2C%20H.%20%282025%2C%20December%2010%29.%20%26lt%3Bi%26gt%3BLandmarks%20in%20the%20Evolution%20of%20Ion%20Chromatography%20%7C%20LCGC%20International%26lt%3B%5C%2Fi%26gt%3B.%20%26lt%3Ba%20class%3D%26%23039%3Bzp-ItemURL%26%23039%3B%20href%3D%26%23039%3Bhttps%3A%5C%2F%5C%2Fwww.chromatographyonline.com%5C%2Fview%5C%2Flandmarks-evolution-ion-chromatography%26%23039%3B%26gt%3Bhttps%3A%5C%2F%5C%2Fwww.chromatographyonline.com%5C%2Fview%5C%2Flandmarks-evolution-ion-chromatography%26lt%3B%5C%2Fa%26gt%3B%26lt%3B%5C%2Fdiv%26gt%3B%5Cn%26lt%3B%5C%2Fdiv%26gt%3B%22%2C%22data%22%3A%7B%22itemType%22%3A%22webpage%22%2C%22title%22%3A%22Landmarks%20in%20the%20Evolution%20of%20Ion%20Chromatography%20%7C%20LCGC%20International%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Hamish%22%2C%22lastName%22%3A%22Small%22%7D%5D%2C%22abstractNote%22%3A%22From%20the%20invention%20of%20eluent%20suppression%20to%20today%26%23039%3Bs%20%26quot%3Bjust%20add%20water%26quot%3B%20concept%2C%20pivotal%20developments%20over%20the%20last%2040%20years%20are%20chronologically%20highlighted%20from%20a%20chemical%20and%20instrumental%20viewpoint.%22%2C%22date%22%3A%222025-12-10T18%3A40%3A03.203Z%22%2C%22DOI%22%3A%22%22%2C%22citationKey%22%3A%22%22%2C%22url%22%3A%22https%3A%5C%2F%5C%2Fwww.chromatographyonline.com%5C%2Fview%5C%2Flandmarks-evolution-ion-chromatography%22%2C%22language%22%3A%22en%22%2C%22collections%22%3A%5B%5D%2C%22dateModified%22%3A%222025-12-10T18%3A41%3A10Z%22%7D%7D%2C%7B%22key%22%3A%22HYKKEQLS%22%2C%22library%22%3A%7B%22id%22%3A21495%7D%2C%22meta%22%3A%7B%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%26lt%3Bdiv%20class%3D%26quot%3Bcsl-bib-body%26quot%3B%20style%3D%26quot%3Bline-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%26quot%3B%26gt%3B%5Cn%20%20%26lt%3Bdiv%20class%3D%26quot%3Bcsl-entry%26quot%3B%26gt%3B%26lt%3Bi%26gt%3BEvolution%20and%20Impact%20of%20Ion-Exchange%20Chromatography%26lt%3B%5C%2Fi%26gt%3B.%20%28n.d.%29.%20Chrom%20Tech%2C%20Inc.%20Retrieved%20December%2010%2C%202025%2C%20from%20%26lt%3Ba%20class%3D%26%23039%3Bzp-ItemURL%26%23039%3B%20href%3D%26%23039%3Bhttps%3A%5C%2F%5C%2Fchromtech.com%5C%2Fblog%5C%2Fevolution-and-impact-of-ion-exchange-chromatography%5C%2F%26%23039%3B%26gt%3Bhttps%3A%5C%2F%5C%2Fchromtech.com%5C%2Fblog%5C%2Fevolution-and-impact-of-ion-exchange-chromatography%5C%2F%26lt%3B%5C%2Fa%26gt%3B%26lt%3B%5C%2Fdiv%26gt%3B%5Cn%26lt%3B%5C%2Fdiv%26gt%3B%22%2C%22data%22%3A%7B%22itemType%22%3A%22webpage%22%2C%22title%22%3A%22Evolution%20and%20Impact%20of%20Ion-Exchange%20Chromatography%22%2C%22creators%22%3A%5B%5D%2C%22abstractNote%22%3A%22Discover%20the%20evolution%20and%20impact%20of%20ion-exchange%20chromatography.%20Learn%20about%20its%20principles%2C%20technical%20aspects%2C%20applications%2C%20and%20innovations%20in%20the%20field.%22%2C%22date%22%3A%22%22%2C%22DOI%22%3A%22%22%2C%22citationKey%22%3A%22%22%2C%22url%22%3A%22https%3A%5C%2F%5C%2Fchromtech.com%5C%2Fblog%5C%2Fevolution-and-impact-of-ion-exchange-chromatography%5C%2F%22%2C%22language%22%3A%22en%22%2C%22collections%22%3A%5B%5D%2C%22dateModified%22%3A%222025-12-10T18%3A38%3A04Z%22%7D%7D%2C%7B%22key%22%3A%22V4FSXLRP%22%2C%22library%22%3A%7B%22id%22%3A21495%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Buie%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%26lt%3Bdiv%20class%3D%26quot%3Bcsl-bib-body%26quot%3B%20style%3D%26quot%3Bline-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%26quot%3B%26gt%3B%5Cn%20%20%26lt%3Bdiv%20class%3D%26quot%3Bcsl-entry%26quot%3B%26gt%3BBuie%2C%20J.%20%28n.d.%29.%20%26lt%3Bi%26gt%3BEvolution%20of%20Chromatography%20Columns%26lt%3B%5C%2Fi%26gt%3B.%20Lab%20Manager.%20Retrieved%20December%2010%2C%202025%2C%20from%20%26lt%3Ba%20class%3D%26%23039%3Bzp-ItemURL%26%23039%3B%20href%3D%26%23039%3Bhttps%3A%5C%2F%5C%2Fwww.labmanager.com%5C%2Fevolution-of-chromatography-columns-18674%26%23039%3B%26gt%3Bhttps%3A%5C%2F%5C%2Fwww.labmanager.com%5C%2Fevolution-of-chromatography-columns-18674%26lt%3B%5C%2Fa%26gt%3B%26lt%3B%5C%2Fdiv%26gt%3B%5Cn%26lt%3B%5C%2Fdiv%26gt%3B%22%2C%22data%22%3A%7B%22itemType%22%3A%22webpage%22%2C%22title%22%3A%22Evolution%20of%20Chromatography%20Columns%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22John%22%2C%22lastName%22%3A%22Buie%22%7D%5D%2C%22abstractNote%22%3A%22The%20history%20of%20chromatography%20dates%20back%20to%20the%20mid-19th%20century%22%2C%22date%22%3A%22%22%2C%22DOI%22%3A%22%22%2C%22citationKey%22%3A%22%22%2C%22url%22%3A%22https%3A%5C%2F%5C%2Fwww.labmanager.com%5C%2Fevolution-of-chromatography-columns-18674%22%2C%22language%22%3A%22en%22%2C%22collections%22%3A%5B%5D%2C%22dateModified%22%3A%222025-12-10T18%3A35%3A38Z%22%7D%7D%2C%7B%22key%22%3A%22U8PXPKWZ%22%2C%22library%22%3A%7B%22id%22%3A21495%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Today%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%26lt%3Bdiv%20class%3D%26quot%3Bcsl-bib-body%26quot%3B%20style%3D%26quot%3Bline-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%26quot%3B%26gt%3B%5Cn%20%20%26lt%3Bdiv%20class%3D%26quot%3Bcsl-entry%26quot%3B%26gt%3BToday%2C%20C.%20%28n.d.%29.%20%26lt%3Bi%26gt%3BDiscovering%20Ion%20Exchange%20Chromatography%26%23x2019%3Bs%20Fascinating%20History%26lt%3B%5C%2Fi%26gt%3B.%20Chromatography%20Today.%20Retrieved%20December%2010%2C%202025%2C%20from%20%26lt%3Ba%20class%3D%26%23039%3Bzp-ItemURL%26%23039%3B%20href%3D%26%23039%3Bhttps%3A%5C%2F%5C%2Fwww.chromatographytoday.com%5C%2Fnews%5C%2Fpreparative%5C%2F33%5C%2Fbreaking-news%5C%2Fdiscovering-ion-exchange-chromatographyrsquos-fascinating-history%5C%2F34442%26%23039%3B%26gt%3Bhttps%3A%5C%2F%5C%2Fwww.chromatographytoday.com%5C%2Fnews%5C%2Fpreparative%5C%2F33%5C%2Fbreaking-news%5C%2Fdiscovering-ion-exchange-chromatographyrsquos-fascinating-history%5C%2F34442%26lt%3B%5C%2Fa%26gt%3B%26lt%3B%5C%2Fdiv%26gt%3B%5Cn%26lt%3B%5C%2Fdiv%26gt%3B%22%2C%22data%22%3A%7B%22itemType%22%3A%22webpage%22%2C%22title%22%3A%22Discovering%20Ion%20Exchange%20Chromatography%5Cu2019s%20Fascinating%20History%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Chromatography%22%2C%22lastName%22%3A%22Today%22%7D%5D%2C%22abstractNote%22%3A%22Ion%20exchange%20chromatography%20%28IEX%29%20works%20by%20separating%20out%20ions%20and%20polar%20molecules%20by%20using%20the%20amount%20of%20charge%20that%20each%20individual%20component%20holds.%20It%20can%20be%20applied%20to%20pretty%20much%20any%20charged%20m...%22%2C%22date%22%3A%22%22%2C%22DOI%22%3A%22%22%2C%22citationKey%22%3A%22%22%2C%22url%22%3A%22https%3A%5C%2F%5C%2Fwww.chromatographytoday.com%5C%2Fnews%5C%2Fpreparative%5C%2F33%5C%2Fbreaking-news%5C%2Fdiscovering-ion-exchange-chromatographyrsquos-fascinating-history%5C%2F34442%22%2C%22language%22%3A%22en%22%2C%22collections%22%3A%5B%5D%2C%22dateModified%22%3A%222025-12-10T18%3A28%3A10Z%22%7D%7D%2C%7B%22key%22%3A%22ZGFFSD5I%22%2C%22library%22%3A%7B%22id%22%3A21495%7D%2C%22meta%22%3A%7B%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%26lt%3Bdiv%20class%3D%26quot%3Bcsl-bib-body%26quot%3B%20style%3D%26quot%3Bline-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%26quot%3B%26gt%3B%5Cn%20%20%26lt%3Bdiv%20class%3D%26quot%3Bcsl-entry%26quot%3B%26gt%3B%26lt%3Bi%26gt%3BIon%20Exchange%20Chromatography%20-%20an%20overview%20%7C%20ScienceDirect%20Topics%26lt%3B%5C%2Fi%26gt%3B.%20%28n.d.%29.%20Retrieved%20December%2010%2C%202025%2C%20from%20%26lt%3Ba%20class%3D%26%23039%3Bzp-ItemURL%26%23039%3B%20href%3D%26%23039%3Bhttps%3A%5C%2F%5C%2Fwww.sciencedirect.com%5C%2Ftopics%5C%2Fbiochemistry-genetics-and-molecular-biology%5C%2Fion-exchange-chromatography%26%23039%3B%26gt%3Bhttps%3A%5C%2F%5C%2Fwww.sciencedirect.com%5C%2Ftopics%5C%2Fbiochemistry-genetics-and-molecular-biology%5C%2Fion-exchange-chromatography%26lt%3B%5C%2Fa%26gt%3B%26lt%3B%5C%2Fdiv%26gt%3B%5Cn%26lt%3B%5C%2Fdiv%26gt%3B%22%2C%22data%22%3A%7B%22itemType%22%3A%22webpage%22%2C%22title%22%3A%22Ion%20Exchange%20Chromatography%20-%20an%20overview%20%7C%20ScienceDirect%20Topics%22%2C%22creators%22%3A%5B%5D%2C%22abstractNote%22%3A%22%22%2C%22date%22%3A%22%22%2C%22DOI%22%3A%22%22%2C%22citationKey%22%3A%22%22%2C%22url%22%3A%22https%3A%5C%2F%5C%2Fwww.sciencedirect.com%5C%2Ftopics%5C%2Fbiochemistry-genetics-and-molecular-biology%5C%2Fion-exchange-chromatography%22%2C%22language%22%3A%22%22%2C%22collections%22%3A%5B%5D%2C%22dateModified%22%3A%222025-12-10T18%3A26%3A10Z%22%7D%7D%2C%7B%22key%22%3A%226574LUS2%22%2C%22library%22%3A%7B%22id%22%3A21495%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Cummins%20et%20al.%22%2C%22parsedDate%22%3A%222017%22%2C%22numChildren%22%3A0%7D%2C%22bib%22%3A%22%26lt%3Bdiv%20class%3D%26quot%3Bcsl-bib-body%26quot%3B%20style%3D%26quot%3Bline-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%26quot%3B%26gt%3B%5Cn%20%20%26lt%3Bdiv%20class%3D%26quot%3Bcsl-entry%26quot%3B%26gt%3BCummins%2C%20P.%20M.%2C%20Rochfort%2C%20K.%20D.%2C%20%26amp%3B%20O%26%23x2019%3BConnor%2C%20B.%20F.%20%282017%29.%20Ion-Exchange%20Chromatography%3A%20Basic%20Principles%20and%20Application.%20In%20D.%20Walls%20%26amp%3B%20S.%20T.%20Loughran%20%28Eds.%29%2C%20%26lt%3Bi%26gt%3BProtein%20Chromatography%26lt%3B%5C%2Fi%26gt%3B%20%28Vol.%201485%2C%20pp.%20209%26%23x2013%3B223%29.%20Springer%20New%20York.%20https%3A%5C%2F%5C%2Fdoi.org%5C%2F10.1007%5C%2F978-1-4939-6412-3_11%26lt%3B%5C%2Fdiv%26gt%3B%5Cn%26lt%3B%5C%2Fdiv%26gt%3B%22%2C%22data%22%3A%7B%22itemType%22%3A%22bookSection%22%2C%22title%22%3A%22Ion-Exchange%20Chromatography%3A%20Basic%20Principles%20and%20Application%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22editor%22%2C%22firstName%22%3A%22Dermot%22%2C%22lastName%22%3A%22Walls%22%7D%2C%7B%22creatorType%22%3A%22editor%22%2C%22firstName%22%3A%22Sin%5Cu00e9ad%20T.%22%2C%22lastName%22%3A%22Loughran%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Philip%20M.%22%2C%22lastName%22%3A%22Cummins%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Keith%20D.%22%2C%22lastName%22%3A%22Rochfort%22%7D%2C%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Brendan%20F.%22%2C%22lastName%22%3A%22O%5Cu2019Connor%22%7D%5D%2C%22abstractNote%22%3A%22%22%2C%22bookTitle%22%3A%22Protein%20Chromatography%22%2C%22date%22%3A%222017%22%2C%22originalDate%22%3A%22%22%2C%22originalPublisher%22%3A%22%22%2C%22originalPlace%22%3A%22%22%2C%22format%22%3A%22%22%2C%22ISBN%22%3A%229781493964109%209781493964123%22%2C%22DOI%22%3A%22%22%2C%22citationKey%22%3A%22%22%2C%22url%22%3A%22http%3A%5C%2F%5C%2Flink.springer.com%5C%2F10.1007%5C%2F978-1-4939-6412-3_11%22%2C%22ISSN%22%3A%22%22%2C%22language%22%3A%22%22%2C%22collections%22%3A%5B%22XAWSHPGP%22%5D%2C%22dateModified%22%3A%222025-12-10T18%3A25%3A14Z%22%7D%7D%2C%7B%22key%22%3A%22XH3A8E6B%22%2C%22library%22%3A%7B%22id%22%3A21495%7D%2C%22meta%22%3A%7B%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%26lt%3Bdiv%20class%3D%26quot%3Bcsl-bib-body%26quot%3B%20style%3D%26quot%3Bline-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%26quot%3B%26gt%3B%5Cn%20%20%26lt%3Bdiv%20class%3D%26quot%3Bcsl-entry%26quot%3B%26gt%3B%26lt%3Bi%26gt%3B28.4%3A%20Partition%20Chromatography%20-%20Chemistry%20LibreTexts%26lt%3B%5C%2Fi%26gt%3B.%20%28n.d.%29.%20Retrieved%20December%206%2C%202025%2C%20from%20%26lt%3Ba%20class%3D%26%23039%3Bzp-ItemURL%26%23039%3B%20href%3D%26%23039%3Bhttps%3A%5C%2F%5C%2Fchem.libretexts.org%5C%2FBookshelves%5C%2FAnalytical_Chemistry%5C%2FInstrumental_Analysis_%28LibreTexts%29%5C%2F28%3A_High-Performance_Liquid_Chromatography%5C%2F28.04%3A_Partition_Chromatography%26%23039%3B%26gt%3Bhttps%3A%5C%2F%5C%2Fchem.libretexts.org%5C%2FBookshelves%5C%2FAnalytical_Chemistry%5C%2FInstrumental_Analysis_%28LibreTexts%29%5C%2F28%3A_High-Performance_Liquid_Chromatography%5C%2F28.04%3A_Partition_Chromatography%26lt%3B%5C%2Fa%26gt%3B%26lt%3B%5C%2Fdiv%26gt%3B%5Cn%26lt%3B%5C%2Fdiv%26gt%3B%22%2C%22data%22%3A%7B%22itemType%22%3A%22webpage%22%2C%22title%22%3A%2228.4%3A%20Partition%20Chromatography%20-%20Chemistry%20LibreTexts%22%2C%22creators%22%3A%5B%5D%2C%22abstractNote%22%3A%22%22%2C%22date%22%3A%22%22%2C%22DOI%22%3A%22%22%2C%22citationKey%22%3A%22%22%2C%22url%22%3A%22https%3A%5C%2F%5C%2Fchem.libretexts.org%5C%2FBookshelves%5C%2FAnalytical_Chemistry%5C%2FInstrumental_Analysis_%28LibreTexts%29%5C%2F28%3A_High-Performance_Liquid_Chromatography%5C%2F28.04%3A_Partition_Chromatography%22%2C%22language%22%3A%22%22%2C%22collections%22%3A%5B%22XAWSHPGP%22%5D%2C%22dateModified%22%3A%222025-12-06T21%3A02%3A23Z%22%7D%7D%2C%7B%22key%22%3A%22AWQU363B%22%2C%22library%22%3A%7B%22id%22%3A21495%7D%2C%22meta%22%3A%7B%22parsedDate%22%3A%222025-12-06%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%26lt%3Bdiv%20class%3D%26quot%3Bcsl-bib-body%26quot%3B%20style%3D%26quot%3Bline-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%26quot%3B%26gt%3B%5Cn%20%20%26lt%3Bdiv%20class%3D%26quot%3Bcsl-entry%26quot%3B%26gt%3BChromatography.%20%282025%29.%20In%20%26lt%3Bi%26gt%3BWikipedia%26lt%3B%5C%2Fi%26gt%3B.%20%26lt%3Ba%20class%3D%26%23039%3Bzp-ItemURL%26%23039%3B%20href%3D%26%23039%3Bhttps%3A%5C%2F%5C%2Fen.wikipedia.org%5C%2Fw%5C%2Findex.php%3Ftitle%3DChromatography%26amp%3Boldid%3D1326037608%26%23039%3B%26gt%3Bhttps%3A%5C%2F%5C%2Fen.wikipedia.org%5C%2Fw%5C%2Findex.php%3Ftitle%3DChromatography%26amp%3Boldid%3D1326037608%26lt%3B%5C%2Fa%26gt%3B%26lt%3B%5C%2Fdiv%26gt%3B%5Cn%26lt%3B%5C%2Fdiv%26gt%3B%22%2C%22data%22%3A%7B%22itemType%22%3A%22encyclopediaArticle%22%2C%22title%22%3A%22Chromatography%22%2C%22creators%22%3A%5B%5D%2C%22abstractNote%22%3A%22In%20chemical%20analysis%2C%20chromatography%20is%20a%20laboratory%20technique%20for%20the%20separation%20of%20a%20mixture%20into%20its%20components.%20The%20mixture%20is%20dissolved%20in%20a%20fluid%20solvent%20%28gas%20or%20liquid%29%20called%20the%20mobile%20phase%2C%20which%20carries%20it%20through%20a%20system%20%28a%20column%2C%20a%20capillary%20tube%2C%20a%20plate%2C%20or%20a%20sheet%29%20on%20which%20a%20material%20called%20the%20stationary%20phase%20is%20fixed.%20As%20the%20different%20constituents%20of%20the%20mixture%20tend%20to%20have%20different%20affinities%20for%20the%20stationary%20phase%20and%20are%20retained%20for%20different%20lengths%20of%20time%20depending%20on%20their%20interactions%20with%20its%20surface%20sites%2C%20the%20constituents%20travel%20at%20different%20apparent%20velocities%20in%20the%20mobile%20fluid%2C%20causing%20them%20to%20separate.%20The%20separation%20is%20based%20on%20the%20differential%20partitioning%20between%20the%20mobile%20and%20the%20stationary%20phases.%20Subtle%20differences%20in%20a%20compound%26%23039%3Bs%20partition%20coefficient%20result%20in%20differential%20retention%20on%20the%20stationary%20phase%20and%20thus%20affect%20the%20separation.%5CnChromatography%20may%20be%20preparative%20or%20analytical.%20The%20purpose%20of%20preparative%20chromatography%20is%20to%20separate%20the%20components%20of%20a%20mixture%20for%20later%20use%2C%20and%20is%20thus%20a%20form%20of%20purification.%20This%20process%20is%20associated%20with%20higher%20costs%20due%20to%20its%20mode%20of%20production.%20Analytical%20chromatography%20is%20done%20normally%20with%20smaller%20amounts%20of%20material%20and%20is%20for%20establishing%20the%20presence%20or%20measuring%20the%20relative%20proportions%20of%20analytes%20in%20a%20mixture.%20The%20two%20types%20are%20not%20mutually%20exclusive.%22%2C%22encyclopediaTitle%22%3A%22Wikipedia%22%2C%22date%22%3A%222025-12-06T18%3A55%3A13Z%22%2C%22ISBN%22%3A%22%22%2C%22DOI%22%3A%22%22%2C%22citationKey%22%3A%22%22%2C%22url%22%3A%22https%3A%5C%2F%5C%2Fen.wikipedia.org%5C%2Fw%5C%2Findex.php%3Ftitle%3DChromatography%26oldid%3D1326037608%22%2C%22language%22%3A%22en%22%2C%22collections%22%3A%5B%5D%2C%22dateModified%22%3A%222025-12-06T20%3A59%3A00Z%22%7D%7D%2C%7B%22key%22%3A%22SXQFZB8P%22%2C%22library%22%3A%7B%22id%22%3A21495%7D%2C%22meta%22%3A%7B%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%26lt%3Bdiv%20class%3D%26quot%3Bcsl-bib-body%26quot%3B%20style%3D%26quot%3Bline-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%26quot%3B%26gt%3B%5Cn%20%20%26lt%3Bdiv%20class%3D%26quot%3Bcsl-entry%26quot%3B%26gt%3B%26lt%3Bi%26gt%3BUnderstanding%20Partition%20Chromatography%3A%20Key%20Principles%20and%20Uses%26lt%3B%5C%2Fi%26gt%3B.%20%28n.d.%29.%20Chrom%20Tech%2C%20Inc.%20Retrieved%20December%206%2C%202025%2C%20from%20%26lt%3Ba%20class%3D%26%23039%3Bzp-ItemURL%26%23039%3B%20href%3D%26%23039%3Bhttps%3A%5C%2F%5C%2Fchromtech.com%5C%2Fblog%5C%2Funderstanding-partition-chromatography-key-principles-and-uses%5C%2F%26%23039%3B%26gt%3Bhttps%3A%5C%2F%5C%2Fchromtech.com%5C%2Fblog%5C%2Funderstanding-partition-chromatography-key-principles-and-uses%5C%2F%26lt%3B%5C%2Fa%26gt%3B%26lt%3B%5C%2Fdiv%26gt%3B%5Cn%26lt%3B%5C%2Fdiv%26gt%3B%22%2C%22data%22%3A%7B%22itemType%22%3A%22webpage%22%2C%22title%22%3A%22Understanding%20Partition%20Chromatography%3A%20Key%20Principles%20and%20Uses%22%2C%22creators%22%3A%5B%5D%2C%22abstractNote%22%3A%22Learn%20the%20key%20principles%20and%20uses%20of%20partition%20chromatography.%20Explore%20its%20fundamentals%2C%20techniques%2C%20and%20applications%20across%20industries.%22%2C%22date%22%3A%22%22%2C%22DOI%22%3A%22%22%2C%22citationKey%22%3A%22%22%2C%22url%22%3A%22https%3A%5C%2F%5C%2Fchromtech.com%5C%2Fblog%5C%2Funderstanding-partition-chromatography-key-principles-and-uses%5C%2F%22%2C%22language%22%3A%22en%22%2C%22collections%22%3A%5B%5D%2C%22dateModified%22%3A%222025-12-06T20%3A58%3A49Z%22%7D%7D%2C%7B%22key%22%3A%22GMLYGF7U%22%2C%22library%22%3A%7B%22id%22%3A21495%7D%2C%22meta%22%3A%7B%22creatorSummary%22%3A%22Today%22%2C%22numChildren%22%3A1%7D%2C%22bib%22%3A%22%26lt%3Bdiv%20class%3D%26quot%3Bcsl-bib-body%26quot%3B%20style%3D%26quot%3Bline-height%3A%202%3B%20padding-left%3A%201em%3B%20text-indent%3A-1em%3B%26quot%3B%26gt%3B%5Cn%20%20%26lt%3Bdiv%20class%3D%26quot%3Bcsl-entry%26quot%3B%26gt%3BToday%2C%20C.%20%28n.d.%29.%20%26lt%3Bi%26gt%3BWhy%20is%20Chromatography%20Called%20Chromatography%3F%26lt%3B%5C%2Fi%26gt%3B%20Chromatography%20Today.%20Retrieved%20December%206%2C%202025%2C%20from%20%26lt%3Ba%20class%3D%26%23039%3Bzp-ItemURL%26%23039%3B%20href%3D%26%23039%3Bhttps%3A%5C%2F%5C%2Fwww.chromatographytoday.com%5C%2Fnews%5C%2Fgc-mdgc%5C%2F32%5C%2Fbreaking-news%5C%2Fwhy-is-chromatography-called-chromatography%5C%2F31178%26%23039%3B%26gt%3Bhttps%3A%5C%2F%5C%2Fwww.chromatographytoday.com%5C%2Fnews%5C%2Fgc-mdgc%5C%2F32%5C%2Fbreaking-news%5C%2Fwhy-is-chromatography-called-chromatography%5C%2F31178%26lt%3B%5C%2Fa%26gt%3B%26lt%3B%5C%2Fdiv%26gt%3B%5Cn%26lt%3B%5C%2Fdiv%26gt%3B%22%2C%22data%22%3A%7B%22itemType%22%3A%22webpage%22%2C%22title%22%3A%22Why%20is%20Chromatography%20Called%20Chromatography%3F%22%2C%22creators%22%3A%5B%7B%22creatorType%22%3A%22author%22%2C%22firstName%22%3A%22Chromatography%22%2C%22lastName%22%3A%22Today%22%7D%5D%2C%22abstractNote%22%3A%22Chromatography%20is%20the%20practice%20of%20separating%20out%20a%20compound%20into%20its%20various%20components%20to%20quantify%20and%20identify%20them.%20This%20is%20achieved%20by%20converting%20a%20solid%20or%20liquid%20into%20a%20gas%2C%20and%20measuring%20the...%22%2C%22date%22%3A%22%22%2C%22DOI%22%3A%22%22%2C%22citationKey%22%3A%22%22%2C%22url%22%3A%22https%3A%5C%2F%5C%2Fwww.chromatographytoday.com%5C%2Fnews%5C%2Fgc-mdgc%5C%2F32%5C%2Fbreaking-news%5C%2Fwhy-is-chromatography-called-chromatography%5C%2F31178%22%2C%22language%22%3A%22en%22%2C%22collections%22%3A%5B%5D%2C%22dateModified%22%3A%222025-12-06T20%3A48%3A18Z%22%7D%7D%5D%7D
Daniel J. Peppe, & Alan L. Deino. (2013).
Dating Rocks and Fossils Using Geologic Methods . Nature, Scitable.
https://www.nature.com/scitable/knowledge/library/dating-rocks-and-fossils-using-geologic-methods-107924044/
Steno, N., & Winter, J. G. (1916).
The prodromus of Nicolaus Steno’s dissertation concerning a solid body enclosed by process of nature within a solid; New York, The Macmillan Company; London, Macmillan and Company, limited.
http://archive.org/details/prodromusnicola00wintgoog
Steno, N., Ferdinando II, G.-D. of T., Accademia della Crusca, publisher, & Burndy Library, donor D. (1669).
Nicolai Stenonis De solido intra solidum naturaliter contento dissertationis prodromus, . Florentiae : Ex typographia sub signo Stellae.
http://archive.org/details/nicolaistenonisd00sten
Murphy, M. A., & Salvador, A. (1999). International Stratigraphic Guide
— An abridged version. Episodes , 22 (4), 255–271.
NORTH AMERICAN STRATIGRAPHIC CODE. (2021).
Stratigraphy ,
18 (3), 153–204.
https://doi.org/10.29041/strat.18.3.01
Striegel, A. M. (2022). Size-Exclusion Chromatography: A Twenty-First Century Perspective.
Chromatographia ,
85 (4), 307–313.
https://doi.org/10.1007/s10337-022-04143-1
Affinity Chromatography | Principles . (n.d.). Cube Biotech. Retrieved December 26, 2025, from
https://cube-biotech.com/our-science/protein-purification/affinity-chromatography/
Yan, Y., Xing, T., Huang, X., Peng, W., Wang, S., & Li, N. (2024). Affinity-Resolved Size Exclusion Chromatography Coupled to Mass Spectrometry: A Novel Tool to Study the Attribute-and-Function Relationship in Therapeutic Monoclonal Antibodies.
Analytical Chemistry ,
96 (29), 11716–11724.
https://doi.org/10.1021/acs.analchem.4c00660
Gjoka, X., Schofield, M., Cvetkovic, A., & Gantier, R. (2014). Combined Protein A and size exclusion high performance liquid chromatography for the single-step measurement of mAb, aggregates and host cell proteins.
Journal of Chromatography. B, Analytical Technologies in the Biomedical and Life Sciences ,
972 , 48–52.
https://doi.org/10.1016/j.jchromb.2014.09.017
London, A. S., Patel, K., Quinn, L., & Lemmerer, M. (2015). Application of coupled affinity-sizing chromatography for the detection of proteolyzed HSA-tagged proteins.
Protein Expression and Purification ,
108 , 80–84.
https://doi.org/10.1016/j.pep.2014.12.005
Corso, G., Mäger, I., Lee, Y., Görgens, A., Bultema, J., Giebel, B., Wood, M. J. A., Nordin, J. Z., & Andaloussi, S. E. (2017). Reproducible and scalable purification of extracellular vesicles using combined bind-elute and size exclusion chromatography.
Scientific Reports ,
7 (1), 11561.
https://doi.org/10.1038/s41598-017-10646-x
Lowe, C. R., & Lowe, C. R. (1985). An introduction to affinity chromatography (3. printing). Elsevier Biomedical Press.
Rodriguez, E. L., Poddar, S., Iftekhar, S., Suh, K., Woolfork, A. G., Ovbude, S., Pekarek, A., Walters, M., Lott, S., & Hage, D. S. (2020). Affinity chromatography: A review of trends and developments over the past 50 years.
Journal of Chromatography B ,
1157 , 122332.
https://doi.org/10.1016/j.jchromb.2020.122332
Burgess, R. R. (2018). A brief practical review of size exclusion chromatography: Rules of thumb, limitations, and troubleshooting.
Protein Expression and Purification ,
150 , 81–85.
https://doi.org/10.1016/j.pep.2018.05.007
Garnett, R. H. T., & Basset, N. C. (2005). Placer Deposits. In Economic Geology 100th Anniversary Volume (pp. 813–843). Society of Economic Geologists (SEG).
Hedenquist, J. W., & Society of Economic Geologists (Eds.). (2005). Economic geology: one hundredth anniversary volume; 1905 - 2005 . Soc. of Economic Geologists.
Jahns, R. H., & Burnham, C. W. (1969). Experimental studies of pegmatite genesis: I. A model for the derivation and crystallization of granitic pegmatites. Economic Geology , 64 (8), 843–864.
Igneous Rocks. (n.d.).
Earth@Home . Retrieved December 17, 2025, from
https://earthathome.org/de/rocks/igneous/
Magma crystallization | Research Starters | EBSCO Research . (n.d.). EBSCO. Retrieved December 17, 2025, from
https://www.ebsco.com
Bowen, N. L. (1928).
The evolution of the igneous rocks . Princeton, Princeton University Press.
http://archive.org/details/evolutionofigneo00bowe
Waseem, U. (2025, September 12).
Crystalline vs Amorphous: Structure, Properties and Engineering Implications . Wevolver.
https://www.wevolver.com/article/crystalline-vs-amorphous-structure-properties-and-engineering-implications
Grace Conyers. (2019, February 25). Make Bismuth Crystals.
Insanitek .
https://insanitek.net/make-bismuth-crystals/
Mettler-Toledo International Inc all rights. (n.d.).
Supersaturation and Crystallization . Retrieved December 15, 2025, from
https://www.mt.com/us/en/home/applications/L1_AutoChem_Applications/L2_Crystallization/Supersaturation_Application.html
Kujan, S. L. (n.d.).
Crystallization from a Supersaturated Solution . Department of Chemistry & Chemical Biology | Rutgers, The State University of New Jersey. Retrieved December 15, 2025, from
https://chem.rutgers.edu/cldf-demos/1031-cldf-demo-crystallization
Supersaturation and Crystallization | Harvard Natural Sciences Lecture Demonstrations . (n.d.). Retrieved December 15, 2025, from
https://sciencedemonstrations.fas.harvard.edu/presentations/supersaturation-and-crystallization
Curling, J. (2025, December 10).
HISTORY OF CHROMATOGRAPHY: Process Chromatography: Five Decades of Innovation | BioPharm International .
https://www.biopharminternational.com/view/history-chromatography-process-chromatography-five-decades-innovation
Ion Exchange
Chromatography: Principles and Methods . (n.d.). GE Healthcare.
Jungbauer, A., & Hahn, R. (2009). Chapter 22 Ion-Exchange Chromatography. In Methods in Enzymology (Vol. 463, pp. 349–371). Elsevier. https://doi.org/10.1016/S0076-6879(09)63022-6
McSweeny, E. (2025, October 17). All Charged Up: The Basics of Ion-Exchange Chromatography [Science Lab Support].
BiteSized Bio .
https://bitesizebio.com/31744/basics-ion-exchange-chromatography/
Ion-Exchange Chromatography: An Easy Introduction to the Basics . (2021, October 15).
https://bitesizebio.com/31744/basics-ion-exchange-chromatography/
The 2 Common Types of Ion Exchange Chromatography: Anion vs. Cation . (n.d.). GoldBio. Retrieved December 10, 2025, from
https://www.goldbio.com/blogs/articles/2-common-types-of-ion-exchange-chromatography-anion-vs-cation
Small, H. (2025, December 10).
Landmarks in the Evolution of Ion Chromatography | LCGC International .
https://www.chromatographyonline.com/view/landmarks-evolution-ion-chromatography
Evolution and Impact of Ion-Exchange Chromatography . (n.d.). Chrom Tech, Inc. Retrieved December 10, 2025, from
https://chromtech.com/blog/evolution-and-impact-of-ion-exchange-chromatography/
Buie, J. (n.d.).
Evolution of Chromatography Columns . Lab Manager. Retrieved December 10, 2025, from
https://www.labmanager.com/evolution-of-chromatography-columns-18674
Ion Exchange Chromatography - an overview | ScienceDirect Topics . (n.d.). Retrieved December 10, 2025, from
https://www.sciencedirect.com/topics/biochemistry-genetics-and-molecular-biology/ion-exchange-chromatography
Cummins, P. M., Rochfort, K. D., & O’Connor, B. F. (2017). Ion-Exchange Chromatography: Basic Principles and Application. In D. Walls & S. T. Loughran (Eds.), Protein Chromatography (Vol. 1485, pp. 209–223). Springer New York. https://doi.org/10.1007/978-1-4939-6412-3_11
Understanding Partition Chromatography: Key Principles and Uses . (n.d.). Chrom Tech, Inc. Retrieved December 6, 2025, from
https://chromtech.com/blog/understanding-partition-chromatography-key-principles-and-uses/
Today, C. (n.d.).
Why is Chromatography Called Chromatography? Chromatography Today. Retrieved December 6, 2025, from
https://www.chromatographytoday.com/news/gc-mdgc/32/breaking-news/why-is-chromatography-called-chromatography/31178