Winter is a time for removing snow, and one fail safe go to is ice. Every winter the road crews bring out the ice trucks to help make driving conditions safer. Homeowners, as well, may salt their drives and sidewalks to prevent injury and accidents on their properties. Sure, we could dust the road and sidewalks with sand to help add grit and friction, but it doesn’t get rid of the dangerous ice so the problem isn’t really solved. So, why does salt work for this unique problem? The answer is in thermodynamics and how a solution can change the freezing temperature.
Freezing versus Melting Points
Any substance has three phases: solid, liquid, and gas. These phases change with temperature and pressure, which is why water can boil under 100ºC (212ºF) at higher altitudes. The air pressure gets lower as we travel higher, and thus the boiling temperature lowers.
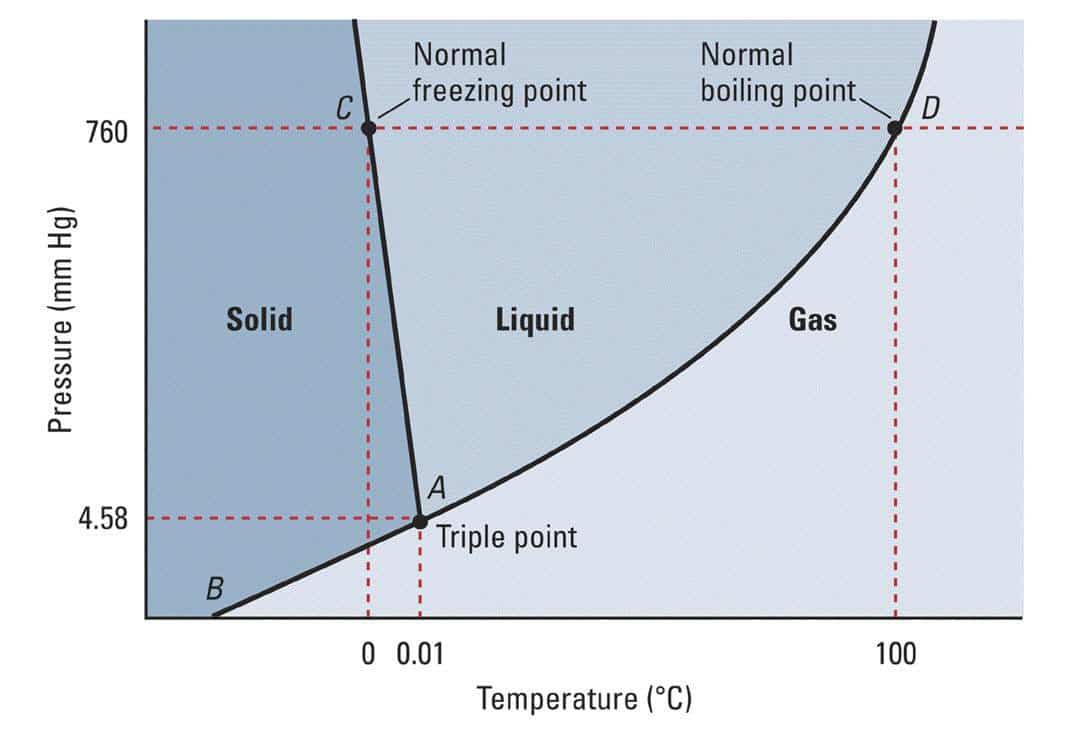
Each substance or solution will have it’s own set of rules that make it useful for predicting and using in labs and beyond. However, when you add something to a solution, it changes to a new set of rules. This is exactly what happens when we add salt to ice: we change it’s phase diagram and set of rules it follows.
Freezing Point Depression
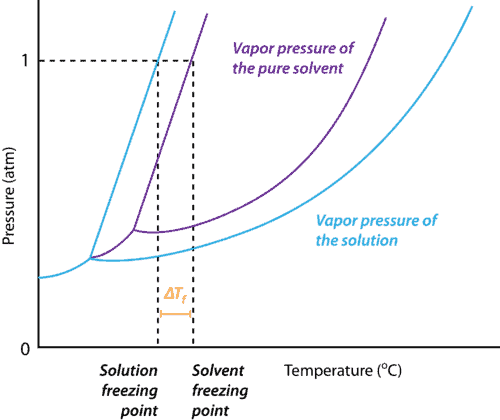
When a solution changes, say from water to salty water, the freezing point changes until the solvent dissolves and washes away. This happens because the solvent is literally different from the original. Take a look at the graph below. The original solvent is shown in purple. It shows that at a certain temperature and pressure the solvent will be a gas, a liquid, or a solid. When any solute is added to it, the solution is fundamentally changed, so it’s graph changes. The blue line represents that change.
Too Cold Is Too Cold
Is there such a thing as too cold for rocks? For rock salt to be halite, no, it can’t be too cold. It can, however, be too cold for the solution of water and salt to create the freezing point depression.
Moderation, As Always, Is Key
Like all other things in life, you can have too much of a good thing. In this case, the salt leaches into the ground and water. The salt used on roads and sidewalks isn’t purified like the salt we use for food, so sodium, chloride, iron, and other impurities can leach into the ground and water supplies. Research done in New England in the Merrimack River watershed points to an average of 11% of the watersheds having an increased salinity and chlorine amount as a result of the salt leaching into the surrounding areas .
Putting it into practice
This is a fun party trick that you can use to teach kids about phase diagrams and why salt helps clear the roads of snow. Grab a piece of string, some ice, a glass of water, and some salt. Roll up your sleeves, and enjoy this simple party trick your little ones can show to everyone.
The FREE worksheet for this hands on activity can be found in our digital resource library. Not a member? You can sign up for free here:

And get more free resources from our library while you’re at it. Drop your email below to get the depth of learning checklist we use AND a few suggestions on how to focus activities for levels of learning.
Bibliography
